filmov
tv
Structure of atom class 10 | planck equation | bohr's model of the atom

Показать описание
ఈ లెసన్ కు సంబంధించి క్రింది అంశాలు సులభమైన పద్ధతిలో ఇంగ్లీష్ మీడియం మరియు తెలుగు మీడియం విద్యార్ధులకు అర్ధమయ్యే విధంగా వివరించబడ్డాయి. ఇవి ఆంధ్ర ప్రదేశ్ మరియు తెలంగాణ రాష్ట్రాలలోని విద్యార్థులను దృష్టిలో ఉంచుకొని వివరించబడ్డాయి
In this chapter the following concepts are explained
Electromagnetic waves, continuous spectrum, discontinuous spectrum, line spectrum, band spectrum, absorption spectrum, emission spectrum, Rutherford atomic model, Bhor atomic model, summer field atomic model, Quantum numbers, electronic configuration, s orbital, p orbital,d orbital,f orbital, pauli's exclusion principle, Hunds rule, Aufbau principle
These lessons are useful for class 10, students, CBSE, students and the students preparing for different competitive examinations like, DSC ,RRB. You can ask doubts in the comment section
please follow this link for all parts of principles of metallurgy
please follow this link for electromagnetism
please follow this link for carbon and its compounds
please follow this link for periodic classification of elements
please follow this link for current electricity
please follow this link for human eye and colourful world
please follow this link for structure of atom
Please follow this link for acids bases and salts
Комментарии
 0:09:49
0:09:49
 0:36:08
0:36:08
 0:05:22
0:05:22
 0:10:37
0:10:37
 0:13:18
0:13:18
 0:18:20
0:18:20
 0:02:20
0:02:20
 0:35:24
0:35:24
 0:01:00
0:01:00
 0:49:06
0:49:06
 0:07:02
0:07:02
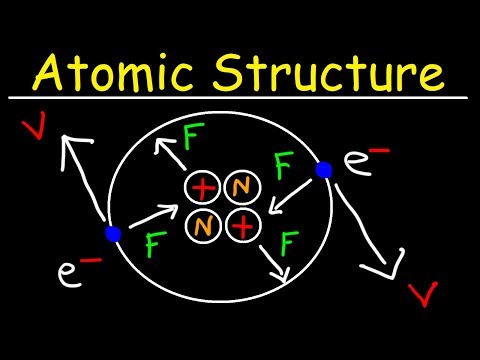 0:11:45
0:11:45
 1:15:45
1:15:45
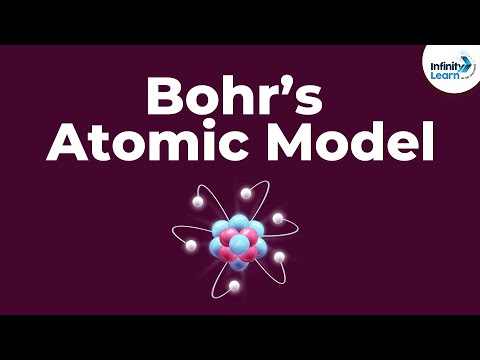 0:05:05
0:05:05
 0:54:21
0:54:21
 2:41:37
2:41:37
 0:04:39
0:04:39
 0:29:21
0:29:21
 0:45:59
0:45:59
 1:12:19
1:12:19
 0:42:14
0:42:14
 1:06:35
1:06:35
 0:30:36
0:30:36
 0:47:45
0:47:45