filmov
tv
Energy & Chemical Change | L 5: Reaction Spontaneity @EasyChemistry4all

Показать описание
#uae #chemistry #grade12 #grade11 #igcse #reactions #chemical_reactions #gibbsfreeenergy #spontaneity
GCSE Chemistry - Exothermic and Endothermic Reactions #43
Exothermic vs Endothermic Chemical Reactions
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool
Energy & Chemistry: Crash Course Chemistry #17
Energy and chemical change Grade 11 Chemistry | Introduction and Overview
Endothermic vs Exothermic Reactions | Energy and Chemical Change | Grade 11
Energy and Chemical Change grade 11: Activation energy, heat of reaction, catalyst and MORE!
What triggers a chemical reaction? - Kareem Jarrah
68 Thermodynamics and Ionic Bonding Exploring Energy Changes in Chemical Reactions
Endothermic and Exothermic Reactions With Potential Energy Diagrams
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Chemical Reactions
Energy and chemical change Heat Of Reaction Grade 11 & 12 Chemistry
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Energy Change Grade 11 Chemistry
Thermochemistry: Heat and Enthalpy
Physical Sciences Grd 11 Energy In Chemical Changes S1
Energy & Chemical Change| L1: Energy @EasyChemistry4all
Chemical Reactions in Biology: Crash Course Biology #26
[4.1] Energy Changes in Chemical Reaction
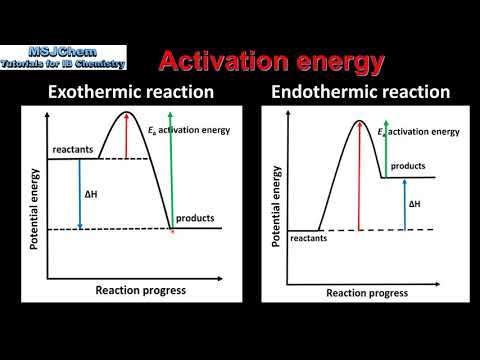
R2.2.4 Activation energy
Chemical Change – Bicarbonate Soda and Vinegar
Physical and Chemical Changes | #aumsum #kids #science #education #children
Grade 11: Energy and Chemical change part 1
Комментарии
 0:05:21
0:05:21
 0:01:49
0:01:49
 0:04:17
0:04:17
 0:09:26
0:09:26
 0:12:56
0:12:56
 0:36:32
0:36:32
 0:10:41
0:10:41
 0:03:46
0:03:46
 0:03:14
0:03:14
 0:10:52
0:10:52
 0:08:12
0:08:12
 0:04:21
0:04:21
 0:07:30
0:07:30
 0:11:27
0:11:27
 0:02:37
0:02:37
 0:04:17
0:04:17
 0:08:05
0:08:05
 0:46:03
0:46:03
 0:13:27
0:13:27
![[4.1] Energy Changes](https://i.ytimg.com/vi/NnCyWPSSbHg/hqdefault.jpg) 0:01:00
0:01:00
 0:03:02
0:03:02
 0:01:39
0:01:39
 0:05:35
0:05:35
 0:27:03
0:27:03