filmov
tv
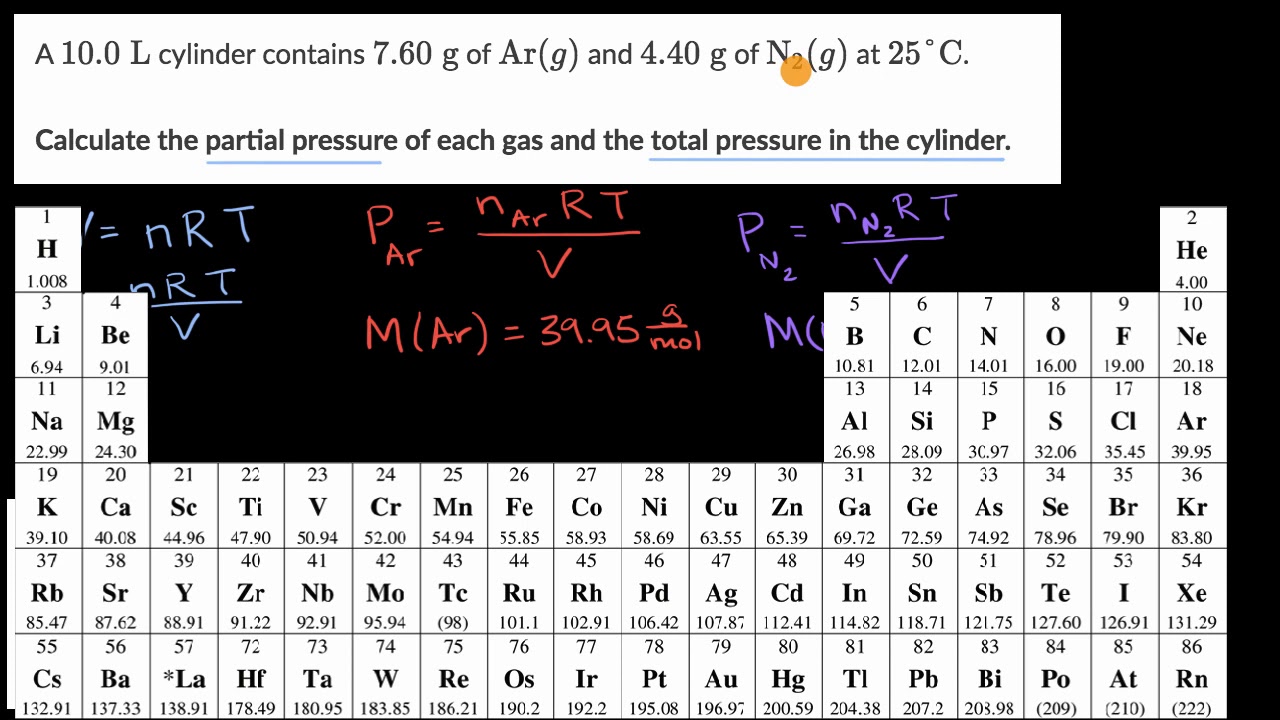
Worked example: Calculating partial pressures | AP Chemistry | Khan Academy
Показать описание
Keep going! Check out the next lesson and practice what you’re learning:
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Worked example: Calculating partial pressures | AP Chemistry | Khan Academy
Worked example: Using the reaction quotient to find equilibrium partial pressures | Khan Academy
Dalton's Law and Partial Pressures
Gas mixtures and partial pressures | AP Chemistry | Khan Academy
Daltons Law | Partial Pressures
Worked example: Calculating an equilibrium constant from initial and equilibrium pressures
Partial Pressures & Vapor Pressure: Crash Course Chemistry #15
Partial Pressures Example Calculation
10.6 Gas Mixtures and Partial Pressures Example #2
Dalton's Law of Partial Pressure | General Chemistry I | 045
10.2 The Combined Gas Law and Dalton's Law of Partial Pressures
Dalton's Law of Partial Pressures + 4 Example Questions
Dalton's Law of Partial Pressures Example Calculation
Partial Pressures (Example)
Partial pressure calculations
Partial Pressure of Gases (Solving for Unknown Pressures)
Example Problem Partial Pressures
Partial pressure - Gas equilibria
⚗️ Partial Pressure and Mole Fractions (Question 1)
⚗️ Total Pressure and Partial Pressures (Question 2)
What is Partial Pressure? | SCUBA THEORY
Partial Pressure
Dalton's Law and Partial Pressures in Scuba Diving
How to work out the partial pressure of a gas
Комментарии
 0:08:03
0:08:03
 0:06:31
0:06:31
 0:06:37
0:06:37
 0:06:23
0:06:23
 0:07:05
0:07:05
 0:04:51
0:04:51
 0:11:55
0:11:55
 0:05:41
0:05:41
 0:01:51
0:01:51
 0:12:22
0:12:22
 0:06:27
0:06:27
 0:09:06
0:09:06
 0:11:23
0:11:23
 0:03:18
0:03:18
 0:06:06
0:06:06
 0:05:50
0:05:50
 0:03:12
0:03:12
 0:08:42
0:08:42
 0:07:54
0:07:54
 0:05:51
0:05:51
 0:04:01
0:04:01
 0:03:33
0:03:33
 0:03:01
0:03:01
 0:07:20
0:07:20