filmov
tv
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry

Показать описание
This video discusses a step-by-step approach to answering equilibrium graph exam questions.
📚Syllabus
• Investigate the effects of temperature, concentration, volume and/or pressure on a system at equilibrium and explain how Le Chatelier’s principle can be used to predict such effects, for example:
– heating cobalt (II) chloride hydrate
– interaction between nitrogen dioxide and dinitrogen tetroxide
– iron(III) thiocyanate and varying concentration of ions (ACSCH095)
How to answer equilibrium graph question? How to answer concentration versus time graph question? Le Chatelier's principle graph question.
📚Syllabus
• Investigate the effects of temperature, concentration, volume and/or pressure on a system at equilibrium and explain how Le Chatelier’s principle can be used to predict such effects, for example:
– heating cobalt (II) chloride hydrate
– interaction between nitrogen dioxide and dinitrogen tetroxide
– iron(III) thiocyanate and varying concentration of ions (ACSCH095)
How to answer equilibrium graph question? How to answer concentration versus time graph question? Le Chatelier's principle graph question.
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry
Equilibrium Graphs: Le Chatelier's Principle (Chemical Equilibrium).
Equilibrium Graphs grade 12: Concentration
Equilibrium Graphs grade 12: Introduction
Equilibrium Graphs Grade 12| How to interpret concentrations time graph
Exam Equilibrium Grade 12
Graphing Equilibrium
Le Chatelier's Principle
Equilibrium Graphs Grade 12| How to interpret pressure vs time graph
Equilibrium Graphs
Equilibrium Graphs Grade 12| How to interpret temperature time graph
Equilibrium Graphs grade 12: Catalyst
GCSE Chemistry - Reversible Reactions and Equilibrium #49
Equilibrium 3: Using Graphs
How to Calculate Equilibrium Price and Quantity (Demand and Supply)
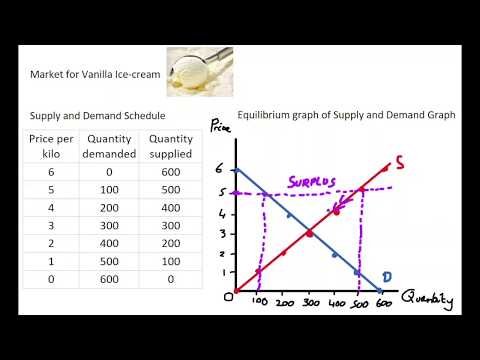
1.7 Equilibrium Market Schedule & Graph
Chemistry | Chemical Change | Chemical Equilibrium Graphs
Equilibrium Constant Grade 12: Exam
Chemistry | Chemical Equilibrium | Kc Calculation | Revision
GRAPH | CHEMICAL EQUILIBRIUM
Physical Sciences 2020: Representing Chemical Equilibrium on a Graph
Changes in equilibrium price and quantity when supply and demand change | Khan Academy
Equilibrium Graphs grade 12 : Interpretation of all factors; concentration, temperature & pressu...
Market equilibrium | Supply, demand, and market equilibrium | Microeconomics | Khan Academy
Комментарии
 0:06:20
0:06:20
 0:07:06
0:07:06
 0:05:32
0:05:32
 0:03:26
0:03:26
 0:09:06
0:09:06
 0:07:48
0:07:48
 0:08:32
0:08:32
 0:26:40
0:26:40
 0:04:26
0:04:26
 0:08:58
0:08:58
 0:06:48
0:06:48
 0:01:17
0:01:17
 0:06:01
0:06:01
 0:06:43
0:06:43
 0:06:08
0:06:08
 0:03:45
0:03:45
 0:27:24
0:27:24
 0:06:48
0:06:48
 0:22:45
0:22:45
 0:01:00
0:01:00
 0:12:16
0:12:16
 0:06:16
0:06:16
 0:28:26
0:28:26
 0:10:17
0:10:17