filmov
tv
Chemistry | Chemical Equilibrium | Kc Calculation | Revision

Показать описание
A Very interesting question on Equilibrium constant and the theoretical understanding of graphs.
You may get in touch with us at:
You may get in touch with us at:
Chemical Equilibria and Reaction Quotients
GCSE Chemistry - Reversible Reactions and Equilibrium #49
Equilibrium: Crash Course Chemistry #28
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
What is chemical equilibrium? - George Zaidan and Charles Morton
Chemical Equilibrium and Equilibrium Constant Chemistry full tutorial video #excellenceacademy
Chemical Equilibrium | Dynamic Equilibrium | Chemistry
Equilibrium Made Easy: How to Solve Chemical Equilibrium Problems
Cambridge AS & A Level Chemistry (9701) || Equilibria || Detailed Explanations
Chemical Equilibrium Full Topic Video
15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
Plus One Chemistry - Equilibrium | Xylem Plus One
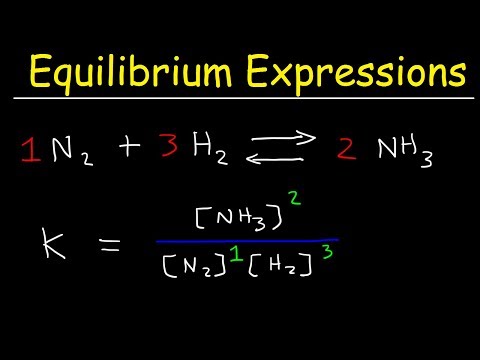
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
What Is Dynamic Equilibrium? | Reactions | Chemistry | FuseSchool
Plus One Chemistry | Equilibrium | Full Chapter | Exam Winner Plus One
Chemical Equilibrium - Introduction
Class 11 Chapter 7 - What is Chemical Equilibrium explained by Tariq Pathan
Plus One Chemistry | Equilibrium | Chapter 6 | Full Chapter | Exam Winner +1
GCSE Chemistry - Equilibrium
Chemical equilibrium|Equilibrium constant|Chemistry
GCSE Chemistry - Le Chatelier's Principle #50 (Higher Tier)
18. Introduction to Chemical Equilibrium
Plus One Chemistry | Equilibrium - Full Chapter Revision | Xylem Plus One
Le Chatelier's Principle
Комментарии
 0:06:48
0:06:48
 0:06:01
0:06:01
 0:10:56
0:10:56
 0:53:22
0:53:22
 0:03:25
0:03:25
 0:26:19
0:26:19
 0:07:09
0:07:09
 0:12:43
0:12:43
 1:35:16
1:35:16
 2:20:00
2:20:00
 0:28:41
0:28:41
 1:25:50
1:25:50
 0:05:24
0:05:24
 0:04:19
0:04:19
 2:29:17
2:29:17
 0:05:33
0:05:33
 0:16:16
0:16:16
 1:25:29
1:25:29
 0:00:15
0:00:15
 0:00:06
0:00:06
 0:03:51
0:03:51
 0:47:51
0:47:51
 2:45:36
2:45:36
 0:26:40
0:26:40