filmov
tv
Kinetics | Using Graphs to Determine Reaction Orders | A level H2 Chem | Making Sense Chem

Показать описание
A LEVEL CHEMISTRY!!
music by:
In today's video, we will be re-visiting at the most important concepts in the chapter Kinetics!
0:00 Introduction
0:13 Recapping Rate equations
1:48 Shapes of graphs
8:43 Pseudo first order reactions
12:05 Determining order through graph
Shapes of conc-time graphs
DO NOT memorise shape of graphs! Use mathematical approach to determine depending on the order of reaction.
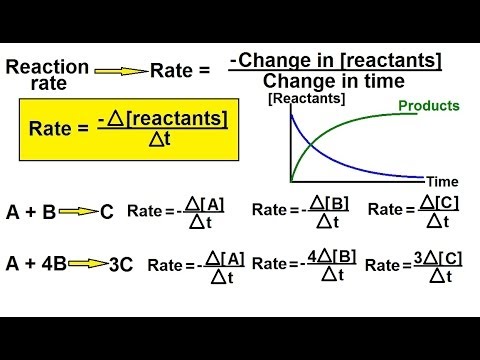
t1/2 is the time required for the concentration of a REACTANT to decrease by HALF.
t1/2 of first order reactions are CONSTANT; value of t1/2 (1st order) = ln2/k
All orders of reactions have half-lives, but only those of first order reactions are CONSTANT.
Pseudo first order reactions
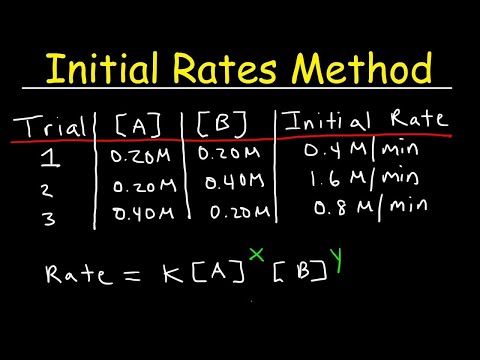
Given a reaction A + B = C with the rate = k[A][B]
To create a pseudo first order reaction wrt A, concentration of all other reagents in rate equation must be CONSTANT (large excess/catalyst).
Group the constants k and [B] together, expressing the new rate constant as k’ (read as k prime)
With [B] constant, the rate equation is now: rate = k' [A] where k' = k[B]
Determining order through graph
For [reactant]-time graphs, shape of the graph determines order: downward sloping straight line (zero order), downward sloping curve (1st or 2nd order)
If the curve is a downward sloping curve, determine the t1/2 of the curve
To find t1/2 of a [reactant]-time curve: first t1/2: time taken for [reactant] to decrease from 100% to 50%; second t1/2: time taken for [reactant] to decrease from 50% to 25%.
If constant t1/2 = 1st order; if non-constant t1/2 = 2nd order.
For [product]-time graph, reaction is only complete when gradient of graph is zero (i.e. graph is flat/plateaus)
Upward sloping straight line (zero order rxn), upward sloping curve (1st or 2nd order - check t1/2 to determine whether 1st or 2nd order)
To find t1/2 of a [product]-time curve: first t1/2: time taken for [product] to increase from 0% to 50%; second t1/2: time taken for [product] to increase from 50% to 75% (50% or 75% of the total amount - 100% - of [product] formed)
Order of reaction wrt product = order of reaction wrt limiting reactant.
Follow us on:
Youtube - Making Sense Tuition Centre
Tik tok - @makingsensechemistry
Instagram - @makingsensechemistry
Facebook - Making Sense, Singapore's Leading Chemistry Tuition
The Best Chemistry tuition!
#indigoeducationgroup
#bestchemistrytuition
#makingsensechemistry
#learningisfun
#videolessons
#educationalvideos
#easyandfunlearning
#reactionkinetics
#rateequations
#orderofreactions
music by:
In today's video, we will be re-visiting at the most important concepts in the chapter Kinetics!
0:00 Introduction
0:13 Recapping Rate equations
1:48 Shapes of graphs
8:43 Pseudo first order reactions
12:05 Determining order through graph
Shapes of conc-time graphs
DO NOT memorise shape of graphs! Use mathematical approach to determine depending on the order of reaction.
t1/2 is the time required for the concentration of a REACTANT to decrease by HALF.
t1/2 of first order reactions are CONSTANT; value of t1/2 (1st order) = ln2/k
All orders of reactions have half-lives, but only those of first order reactions are CONSTANT.
Pseudo first order reactions
Given a reaction A + B = C with the rate = k[A][B]
To create a pseudo first order reaction wrt A, concentration of all other reagents in rate equation must be CONSTANT (large excess/catalyst).
Group the constants k and [B] together, expressing the new rate constant as k’ (read as k prime)
With [B] constant, the rate equation is now: rate = k' [A] where k' = k[B]
Determining order through graph
For [reactant]-time graphs, shape of the graph determines order: downward sloping straight line (zero order), downward sloping curve (1st or 2nd order)
If the curve is a downward sloping curve, determine the t1/2 of the curve
To find t1/2 of a [reactant]-time curve: first t1/2: time taken for [reactant] to decrease from 100% to 50%; second t1/2: time taken for [reactant] to decrease from 50% to 25%.
If constant t1/2 = 1st order; if non-constant t1/2 = 2nd order.
For [product]-time graph, reaction is only complete when gradient of graph is zero (i.e. graph is flat/plateaus)
Upward sloping straight line (zero order rxn), upward sloping curve (1st or 2nd order - check t1/2 to determine whether 1st or 2nd order)
To find t1/2 of a [product]-time curve: first t1/2: time taken for [product] to increase from 0% to 50%; second t1/2: time taken for [product] to increase from 50% to 75% (50% or 75% of the total amount - 100% - of [product] formed)
Order of reaction wrt product = order of reaction wrt limiting reactant.
Follow us on:
Youtube - Making Sense Tuition Centre
Tik tok - @makingsensechemistry
Instagram - @makingsensechemistry
Facebook - Making Sense, Singapore's Leading Chemistry Tuition
The Best Chemistry tuition!
#indigoeducationgroup
#bestchemistrytuition
#makingsensechemistry
#learningisfun
#videolessons
#educationalvideos
#easyandfunlearning
#reactionkinetics
#rateequations
#orderofreactions
 0:09:55
0:09:55
 0:09:21
0:09:21
 0:17:57
0:17:57
 0:34:53
0:34:53
 0:05:52
0:05:52
 0:09:10
0:09:10
 0:48:46
0:48:46
 0:07:13
0:07:13
 0:05:59
0:05:59
 0:06:41
0:06:41
 0:03:41
0:03:41
 0:15:34
0:15:34
 0:22:18
0:22:18
 0:12:29
0:12:29
 0:04:31
0:04:31
 0:04:49
0:04:49
 0:05:35
0:05:35
 0:08:57
0:08:57
 0:04:23
0:04:23
 0:06:27
0:06:27
 0:03:27
0:03:27
 0:32:08
0:32:08
 0:31:50
0:31:50
 0:14:53
0:14:53