filmov
tv
Writing Formulas for Ionic Compounds (Introduction)

Показать описание
Q1. Write the formula for the ionic compound that forms between aluminum and oxygen.
Step 1:
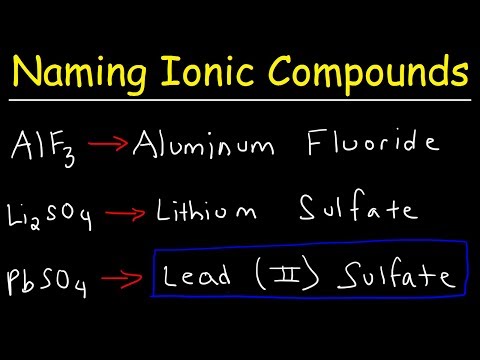
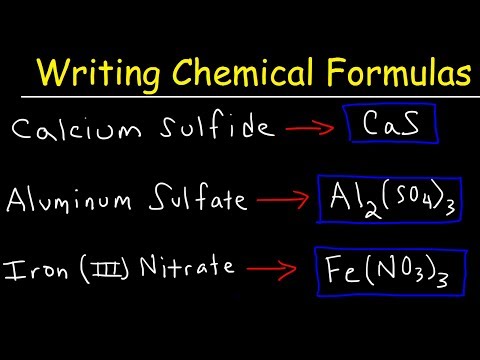
Write the symbol for the metal cation and its charge followed by the symbol for the nonmetal anion and its charge. Determine charges from the element’s group number in the periodic table.
Step 2:
Adjust the subscript on each cation and anion to balance the overall charge.
Step 3:
Check that the sum of the charges of the cations equals the sum of the charges of the anions.
Q2. Write the formula for the compound formed between potassium and sulfur.
 0:10:22
0:10:22
 0:04:39
0:04:39
 0:11:44
0:11:44
 0:14:26
0:14:26
 0:07:21
0:07:21
 0:05:30
0:05:30
 0:13:33
0:13:33
 0:05:05
0:05:05
 0:09:56
0:09:56
 0:04:48
0:04:48
 0:10:41
0:10:41
 0:10:32
0:10:32
 0:16:52
0:16:52
 0:09:17
0:09:17
 0:06:47
0:06:47
 0:03:23
0:03:23
 0:11:21
0:11:21
 0:05:44
0:05:44
 0:06:08
0:06:08
 0:11:44
0:11:44
 0:01:33
0:01:33
 0:08:25
0:08:25
 0:03:48
0:03:48
 0:02:52
0:02:52