filmov
tv
Alcohols to Alkyl Chlorides, Part 4: Phosphorus Chlorides

Показать описание
This presentation is about the conversion of alcohols into alkyl chlorides by means of phosphorus chlorides as deoxychlorinating reagents. These are highly selective and reactive, and easy to handle, and are therefore often used for small scale laboratory preparations. For larger scale preparations, however, phosphorus-based reagents are less popular, because the disposal of phosphorus waste can be difficult.
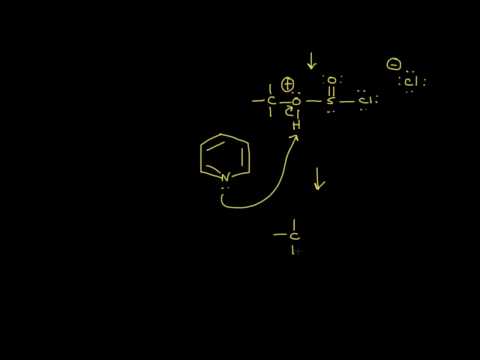
Particularly well suited for the preparation of alkyl chlorides from sensitive alcohols are phosphorus pentachloride and mixtures of triaryl- or trialkylphosphines with a chlorinating reagent. In both instances the deoxychlorinating phosphorus derivative may be a chlorophosphonium salt or a pentavalent phosphorus derivative, depending on the solvent and the precise chlorinating agent chosen.
Suitable chlorinating reagents for the activation of phosphines include elemental chlorine, carbon tetrachloride, SO2Cl2, N-chloroimides, other perchloroalkanes, chloroketones, and trichloroacetonitrile. Particularly useful are carbon tetrachloride or hexachloroethane, because these reagents chlorinate few other functional groups in addition to phosphines.
PCl5 is a solid prepared by chlorination of PCl3 with chlorine. It is often used as solution in POCl3, into which it is converted during the deoxychlorination reaction. In large scale preparations, the POCl3 formed can then be recovered after the reaction by distillation.
Further examples:
PPh3 + chlorinating reagents:
Cl2: Org. Syn. 2010, 87, 299, 317.
- CCl4: J. Org. Chem. 1983, 48, 3721-3728; 1972, 37, 1466.
- C2Cl6: Synthesis 1983, 139-141.- iPr2NCl: Synlett 2000, 671-673.- N-Chlorosuccinimide (NCS): J. Am. Chem. Soc. 2001, 123, 3603-3604.
trichloroisocyanuric acid: Syn. Comm. 2002, 32, 2691-2694.
hexachloroacetone: J. Org. Chem. 1981, 46, 824-825.
POCl3: Chem. Pharm. Bull. 1986, 34, 2799-2809; 1982, 30, 610-614.
PCl5: J. Biol. Chem. 1971, 246, 6855-6866 (serine-OMe); J. Chem. Soc. 1953, 1709-1715.
Particularly well suited for the preparation of alkyl chlorides from sensitive alcohols are phosphorus pentachloride and mixtures of triaryl- or trialkylphosphines with a chlorinating reagent. In both instances the deoxychlorinating phosphorus derivative may be a chlorophosphonium salt or a pentavalent phosphorus derivative, depending on the solvent and the precise chlorinating agent chosen.
Suitable chlorinating reagents for the activation of phosphines include elemental chlorine, carbon tetrachloride, SO2Cl2, N-chloroimides, other perchloroalkanes, chloroketones, and trichloroacetonitrile. Particularly useful are carbon tetrachloride or hexachloroethane, because these reagents chlorinate few other functional groups in addition to phosphines.
PCl5 is a solid prepared by chlorination of PCl3 with chlorine. It is often used as solution in POCl3, into which it is converted during the deoxychlorination reaction. In large scale preparations, the POCl3 formed can then be recovered after the reaction by distillation.
Further examples:
PPh3 + chlorinating reagents:
Cl2: Org. Syn. 2010, 87, 299, 317.
- CCl4: J. Org. Chem. 1983, 48, 3721-3728; 1972, 37, 1466.
- C2Cl6: Synthesis 1983, 139-141.- iPr2NCl: Synlett 2000, 671-673.- N-Chlorosuccinimide (NCS): J. Am. Chem. Soc. 2001, 123, 3603-3604.
trichloroisocyanuric acid: Syn. Comm. 2002, 32, 2691-2694.
hexachloroacetone: J. Org. Chem. 1981, 46, 824-825.
POCl3: Chem. Pharm. Bull. 1986, 34, 2799-2809; 1982, 30, 610-614.
PCl5: J. Biol. Chem. 1971, 246, 6855-6866 (serine-OMe); J. Chem. Soc. 1953, 1709-1715.
 0:08:26
0:08:26
 0:11:32
0:11:32
 0:06:24
0:06:24
 0:12:06
0:12:06
 0:05:15
0:05:15
 0:11:00
0:11:00
 0:07:27
0:07:27
 0:09:20
0:09:20
 0:08:54
0:08:54
 0:05:48
0:05:48
 0:07:57
0:07:57
 0:14:56
0:14:56
 0:04:36
0:04:36
 0:12:37
0:12:37
 0:27:41
0:27:41
 0:14:28
0:14:28
 0:37:34
0:37:34
 0:04:49
0:04:49
 0:16:30
0:16:30
 0:26:56
0:26:56
 0:11:09
0:11:09
 0:40:16
0:40:16
 0:00:57
0:00:57
 0:11:16
0:11:16