filmov
tv
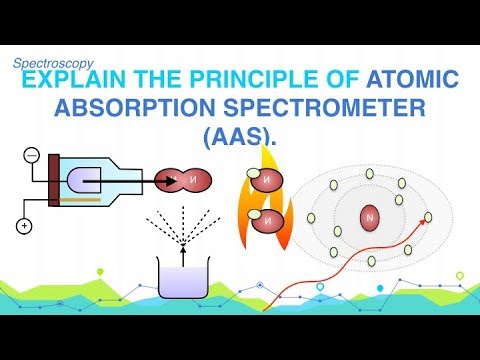
Explain the Principle of Atomic Absorption Spectrometer (AAS)

Показать описание
When the salt soln. is put into the flame first solvent is vaporized the tiny particles of solute molecules are obtained which on further heating in the flame are converted into gaseous molecules the molecules dissociate into atoms. Some of the atoms are exited unexcited atoms will absorb their characteristic radiation. A.A.S. is concerned with this part of light (radiations) which are absorbed by the unexcited atoms present in the ground state. This absorption depends on no of unexcited atoms present and hence, is independent of the flame temp. It is possible, if the course of radiations is made-up of the same element which is going to be analyzed. For e.g. if the sample solution of NaCl is to be analyzed, source of radiations must be Na-Vapors.
Where I0 = Intensity of radiations incident.
It = Intensity of radiations transmitted.
K = Characteristics Constant,
L = Path length of flame in cm.
No = No of atoms in the ground state.
The absorption of radiations follows the Beer-Lamberts Law. As each element absorb the radiations of its own characteristics therefore separate source of radiations is required for each element.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
Where I0 = Intensity of radiations incident.
It = Intensity of radiations transmitted.
K = Characteristics Constant,
L = Path length of flame in cm.
No = No of atoms in the ground state.
The absorption of radiations follows the Beer-Lamberts Law. As each element absorb the radiations of its own characteristics therefore separate source of radiations is required for each element.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
Комментарии
 0:02:59
0:02:59
 0:03:05
0:03:05
 0:01:44
0:01:44
 0:11:57
0:11:57
 0:11:45
0:11:45
 0:08:01
0:08:01
 0:10:53
0:10:53
 0:08:42
0:08:42
 0:13:00
0:13:00
 0:00:58
0:00:58
 0:03:52
0:03:52
 0:13:06
0:13:06
 0:11:19
0:11:19
 0:21:44
0:21:44
 0:01:23
0:01:23
 0:06:07
0:06:07
 0:09:19
0:09:19
 0:13:46
0:13:46
 0:14:04
0:14:04
 0:07:53
0:07:53
 0:05:08
0:05:08
 0:10:19
0:10:19
 0:12:15
0:12:15
 0:06:27
0:06:27