filmov
tv
Basic Atomic Structure | Radiology Physics Course #1

Показать описание
*High yield radiology physics past paper questions with video answers*
Perfect for testing yourself prior to your radiology physics exam 👇
=========================
*I have also created two RADIOPAEDIA LEARNING PATHWAYS*
WHAT’S INCLUDED?
✅This YouTube series Ad free
✅Constantly updated Radiopaedia articles
✅Summary slides
✅Key take home bullet points throughout
✅Multiple review quizzes
✅Short answer review questions
✅Official Radiopaedia course completion certificate
=========================
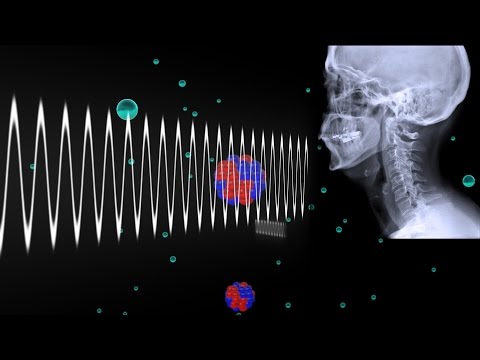
Let's look at the Rutherford-Bohr model of the atom. We review the nucleus (protons and neutrons) and the orbiting electrons, briefly discussing the concept of energy levels or electron shells. Next we discuss the basic notation of nuclides before looking at the classification of nuclides (isotopes, isobars, isotones and isomers).
=========================
*Not sure if the question banks are for you?*
If you're here, you're likely studying for a radiology physics exam. I've spent the last few months collating past papers from multiple different countries selecting the most commonly asked questions. You'll be surprised how often questions repeat themselves!
The types of questions asked in FRCR, RANZCR AIT, ARRT, FC Rad Diag (SA), ABR qualifying Core Physics and MICR part 1 are surprisingly similar and the key concepts remain the same throughout. I've taken the most high-yield questions and answered them in video format so that I can take you through why certain answers are correct and others are not.
Happy studying,
Michael
#radiology #radres #FOAMrad #FOAMed
Perfect for testing yourself prior to your radiology physics exam 👇
=========================
*I have also created two RADIOPAEDIA LEARNING PATHWAYS*
WHAT’S INCLUDED?
✅This YouTube series Ad free
✅Constantly updated Radiopaedia articles
✅Summary slides
✅Key take home bullet points throughout
✅Multiple review quizzes
✅Short answer review questions
✅Official Radiopaedia course completion certificate
=========================
Let's look at the Rutherford-Bohr model of the atom. We review the nucleus (protons and neutrons) and the orbiting electrons, briefly discussing the concept of energy levels or electron shells. Next we discuss the basic notation of nuclides before looking at the classification of nuclides (isotopes, isobars, isotones and isomers).
=========================
*Not sure if the question banks are for you?*
If you're here, you're likely studying for a radiology physics exam. I've spent the last few months collating past papers from multiple different countries selecting the most commonly asked questions. You'll be surprised how often questions repeat themselves!
The types of questions asked in FRCR, RANZCR AIT, ARRT, FC Rad Diag (SA), ABR qualifying Core Physics and MICR part 1 are surprisingly similar and the key concepts remain the same throughout. I've taken the most high-yield questions and answered them in video format so that I can take you through why certain answers are correct and others are not.
Happy studying,
Michael
#radiology #radres #FOAMrad #FOAMed
Комментарии
 0:05:08
0:05:08
 0:10:23
0:10:23
 0:06:51
0:06:51
 0:01:30
0:01:30
 0:10:33
0:10:33
 0:09:42
0:09:42
 0:02:12
0:02:12
 0:01:29
0:01:29
 0:04:26
0:04:26
 0:07:04
0:07:04
 0:12:15
0:12:15
 0:08:21
0:08:21
 0:42:44
0:42:44
 0:06:01
0:06:01
 0:03:19
0:03:19
 0:21:24
0:21:24
 0:23:40
0:23:40
 0:02:02
0:02:02
 0:44:52
0:44:52
 0:00:32
0:00:32
 0:15:37
0:15:37
 0:04:59
0:04:59
 0:39:49
0:39:49
 0:01:09
0:01:09