filmov
tv
Splitting of d-orbitals in various complexes

Показать описание
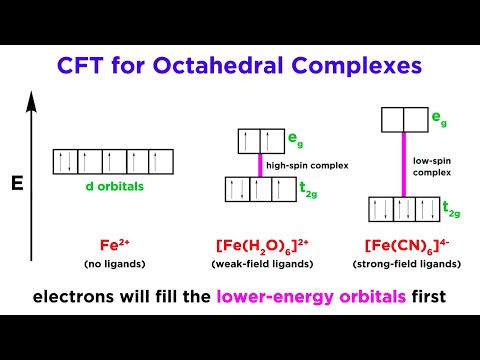
d-Orbitals of a metal ion in free state have the same energy. These d-orbitals with the same enrggy are called degenerate d-orbitals. In the metal complexes the degeneracy is lost because there exist two or more than two energy levels. This is called splitting of d-orbitals. The splitting pattern of d-orbitals depends upon the geometry of the complex. In this lecture, splitting of d-orbital in the complexes with different geometries have discussed in detail.
Splitting of d-orbitals in various complexes
d-orbital splitting - Crystal Field Theory (A-Level IB Chemistry)
Crystal Field Theory
Why does d-orbital Splitting Happen?
d orbital splitting
Crystal Field Theory
L15B d orbital splitting
Splitting Of d Orbitals In Various Geometry | JAM/CSIR-NET/GATE/JEE etc | Akacademy
What are the Orbitals in principal quantum number 2? #chemistry
Crystal Field Theory | Easy Trick
CRYSTAL FIELD SPLITTING IN TETRAHEDRAL COMPLEX | CFT | LFT | SPLITTING OF d ORBITALS
Trick ( splitting of d orbital in different environments) by AP sir AIR 48 CSIR net
d orbitals splitting in different crystal fields for different coordination numbers | 15 Minute E 38
crystal field splitting in tetrahedral complexes #ytshorts #youtubeshorts
Splitting Of d-orbitals | Octahedral, Square planar, tetrahedral| IIT-JEE |NEET |IIT-JAM |CSIR-NET
21.4 Crystal Field Theory | General Chemistry
d-orbital splitting patterns in Trigonal bipyramidal, Square pyramidal, Linear complexes.
Splitting of d-orbitals in tetrahedral,square plannar, sq.pyramidal and trigonal prismatic complexes
M. Sc Chemistry, Inorganic Chemistry, d-orbital Splitting Pattern in Octahedral Complexes
Factors affecting magnitude of d-Orbital splitting (∆)
Crystal field Splitting of d orbitals in Tetragonal and square planar complexes
Splitting of d orbitals in Tetrahedral complexes | Crystal Field Theory
Crystal field splitting in various geometry ll Coordination Chemistry
28. Transition Metals: Crystal Field Theory Part I
Комментарии
 0:13:41
0:13:41
 0:10:20
0:10:20
 0:07:42
0:07:42
 0:02:52
0:02:52
 0:14:27
0:14:27
 0:21:56
0:21:56
 0:08:32
0:08:32
 0:05:53
0:05:53
 0:00:44
0:00:44
 0:15:32
0:15:32
 0:06:33
0:06:33
 0:07:20
0:07:20
 0:25:04
0:25:04
 0:00:31
0:00:31
 0:00:13
0:00:13
 0:23:25
0:23:25
 0:04:32
0:04:32
 0:38:07
0:38:07
 0:23:32
0:23:32
 0:08:34
0:08:34
 0:19:49
0:19:49
 0:05:36
0:05:36
 0:13:55
0:13:55
 0:53:35
0:53:35