filmov
tv
Carbon Allotropes | A Level Chemistry | OCR, AQA, Edexcel

Показать описание
Our A-Level Chemistry Experts are here to help you ace A-Level Chemistry!
This week we are revising Carbon Allotropes
A-Level Chemistry can be tough but fortunately we’ve made this tutorial to help you score the A* you need for questions on everything to do with Carbon Allotropes.
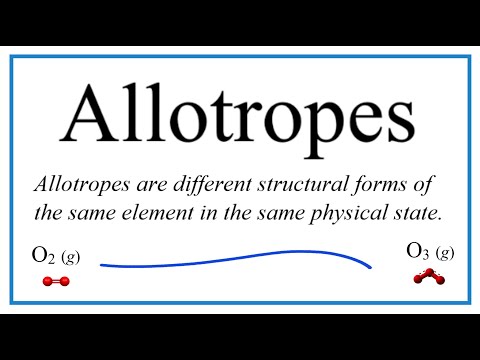
Allotropes are different molecular or crystalline forms of the same element, resulting in different physical properties.
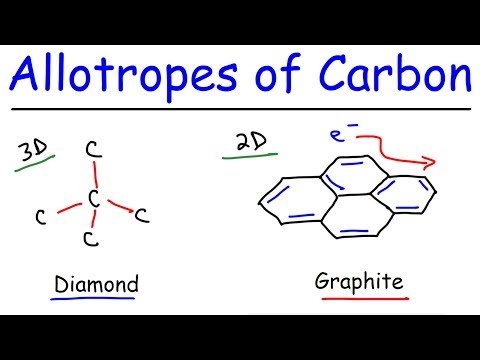
Carbon is an example of an element that exists as different allotropes. Each carbon atom has an outermost electron shell with 4 electrons. This means that each carbon atom needs to gain 4 more electrons to gain a full outer shell of electrons. The carbon atoms gain a full outer shell through covalent bonds in which they form two different macromolecular structures: diamond and graphite.
In diamond, each carbon atom forms four covalent bonds with four other carbon atoms. In diamond, each carbon shares electrons with four other carbon atoms. This means that each carbon atom forms a single covalent bond with four other carbon atoms.
Diamond is a very hard substance due to its strong covalent bonds. The strong covalent bonds in diamond means that it is very difficult to break. It is actually known to be the hardest naturally occurring substance found on Earth. As a result of its hardness, diamond is often used to coat drill bits.
Diamond has a high melting and boiling point. The covalent bonds in diamond are very strong, therefore a large amount of energy is needed to break them.
Diamond is a good conductor of heat. Diamond is a good thermal conductor because of the strong covalent bonds it consists of. This means that when you heat the diamond, the vibrations of thermal energy are rapidly transferred through the substance.
Diamond is insoluble in both water and in organic solvents. Diamond does not dissolve in any solvent. This is because the attraction between the carbon atoms in diamond by covalent bonds is a lot stronger than the attraction that could occur between the solvent molecules and carbon atoms in diamond.
Diamond is a poor conductor of electricity. Diamond cannot conduct electric because the outer electrons found in each carbon atom are fixated between the atoms in covalent bonds. This means there are no free electrons that can move around and carry charge.
In graphite, each carbon atom forms three covalent bonds with three other carbon atoms. In graphite, each carbon shares electrons with three other carbon atoms. This means that each carbon atom has one outer electron that is not involved in a covalent bond. This ‘fourth’ electron becomes delocalised and is free to move around.
The carbon atoms in graphite are organised into sheets of hexagons. In graphite, the carbon atoms are arranged into sheets which means that graphite has a layer structure. The sheets are arranged into layers and the layers are joined together by weak intermolecular forces called ‘van Der Waals forces’.
Graphite is soft slippery substance because it consists of layers that can slide. Unlike diamond, graphite is arranged in layers and sheets of carbon atoms. The layers in graphite can easily slide over each other because there are weak intermolecular forces holding them together. Due to its slippery nature, graphite act be used in pencils and as a dry lubricant.
Graphite has a high melting and boiling point. The covalent bonds in graphite are very strong, therefore a large amount of energy is needed to break them.
Graphite is insoluble in both water and in organic solvents. Graphite does not dissolve in any solvent. This is because the attraction between the carbon atoms in graphite by covalent bonds is a lot stronger than the attraction that could occur between the solvent molecules and carbon atoms in graphite.
Graphite is a good conductor of electricity. Graphite can conduct electricity because it contains delocalised electrons which are free to move between the sheets of carbon atoms and carry charge.
Graphite has a low density because the distance between the layers is large. As the layers in graphite are held together by weak intermolecular forces, the layers are far apart.
#ALevelChemistry #ALevelBiology #Biology #Chemistry #StudyMind
 0:03:22
0:03:22
 0:06:33
0:06:33
 0:07:26
0:07:26
 0:03:07
0:03:07
 0:17:10
0:17:10
 0:03:23
0:03:23
 0:04:28
0:04:28
 0:03:14
0:03:14
 0:02:22
0:02:22
 0:11:16
0:11:16
 0:04:07
0:04:07
 0:06:22
0:06:22
 0:36:39
0:36:39
 0:04:46
0:04:46
 0:05:40
0:05:40
 0:05:41
0:05:41
 0:01:24
0:01:24
 0:51:12
0:51:12
 0:06:53
0:06:53
 0:44:26
0:44:26
 0:01:00
0:01:00
 0:09:20
0:09:20
 0:03:46
0:03:46
 0:26:14
0:26:14