filmov
tv
Practising drawing complex ions from www.ChemistryTuition.Net

Показать описание
Practising drawing complex ions from www.ChemistryTuition.Net
Complex Ions, Ligands, & Coordination Compounds, Basic Introduction Chemistry
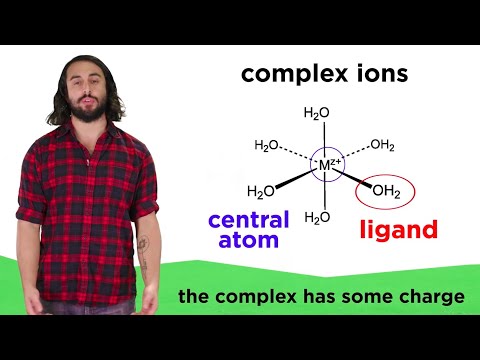
Complex Ion Formation
More practice at drawing complex ions from www.ChemistryTuition.Net
Draw the isomers of [Co(NH3)3(NO2)3] complex
Transition metal complex ions
Naming Coordination Compounds - Chemistry
Chem 123 - Complex Ion Extra Practice
21.2 Naming Complex Ions and Coordination Compounds | General Chemistry
Transition Metals - Complex Shapes and Isomers|AQA A Level Chemistry
S3.1.8 Complex ions (HL)
How To Draw Lewis Structures
Coordination Compounds: Geometry and Nomenclature
Tricky Question -complex ions
Drawing Complex Lewis Structures and Formal Atom Charges
21.1 Introduction to Coordination Chemistry | General Chemistry
21.3 Isomers in Coordination Chemistry | General Chemistry
Shapes of complex ions from www.ChemistryTuition.Net
How to Name Coordination Compounds and Complex Ions Explained Simply
Transition Metal Complexes (A2 Chemistry)
drawing complexes 1 - monodentate
13.1 Complex ions (HL)
Isomers in inorganic complexes
Complex Ions
Комментарии
 0:12:27
0:12:27
 0:13:42
0:13:42
 0:04:06
0:04:06
 0:03:28
0:03:28
 0:04:23
0:04:23
 0:11:59
0:11:59
 0:10:45
0:10:45
 0:05:58
0:05:58
 0:13:51
0:13:51
 0:48:40
0:48:40
 0:04:22
0:04:22
 0:11:50
0:11:50
 0:09:15
0:09:15
 0:08:08
0:08:08
 0:07:33
0:07:33
 0:13:12
0:13:12
 0:24:17
0:24:17
 0:06:09
0:06:09
 0:11:09
0:11:09
 0:16:17
0:16:17
 0:12:12
0:12:12
 0:04:05
0:04:05
 0:15:44
0:15:44
 0:05:48
0:05:48