filmov
tv
Pauling's Rule 2 (Part C): Anisodesmic compunds, CaCO3 example

Показать описание
Discusses the case of unequal bonding in minerals (so-called "aniosdesmic compounds") and how Pauling's rules can be used to predict the dissolution or reaction behavior of such compounds, using calcite, CaCO3 as an exampl (by Keith Putirka)
Pauling's Rule 2 (part C)
MSE 403 S21 Lecture 4 - Module 2 - Pauling's Rules
Crystal Structure, Coordination Number & Polyhedra, Pauling's Rules, Bonding- Mineralogy | ...
Pauling's rule
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configur...
Pauling's Rule 2 (Part A): electrostatic valence principle (SiO2 as an example)
Pauling's Rule 2 (part B): garnet example
Pauling's Rules 3 and 4: minimizing cation repulsions
Pauling and Mulliken Electronegativity (Detailed Video With All The Formulas & Derivations)
Pauling's Rule 5
Pauling's Recommendations for Vitamin C and Lysine
Pauling's rules 1-5 overview
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
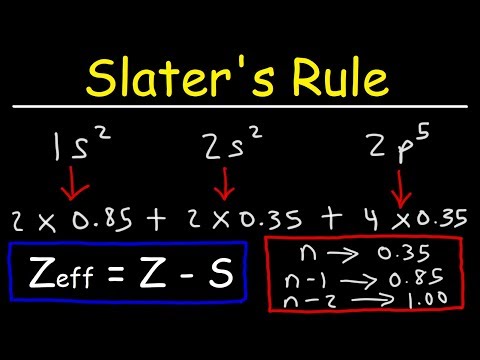
How To Use Slater's Rule to Estimate The Effective Nuclear Charge
Polar Covalent Bonds: Who was Linus Pauling?
How To Calculate The Effective Nuclear Charge of an Electron
SOLUTIONS to Linus Pauling's 'General Chemistry' - Chapter 2 - Part 2 -- Problems 3, ...
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds
PAULING's METHOD TE DETERMINE IONIC RADII/XI/U-3/EM
M-01.b. Pauling's rules
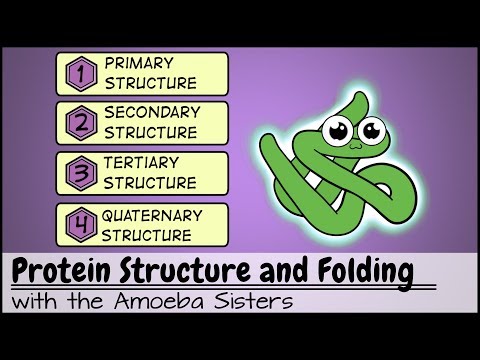
Protein Structure and Folding
Pauling's Rule 1: Coordination principle Radius Ratios
On the Pauling's `EN` scale, the element next to `F` is `'______'`
Anisodesmic Crystal
Комментарии
 0:05:47
0:05:47
 0:17:07
0:17:07
 0:29:49
0:29:49
 0:27:57
0:27:57
 0:05:24
0:05:24
 0:07:39
0:07:39
 0:06:25
0:06:25
 0:07:52
0:07:52
 0:08:32
0:08:32
 0:07:04
0:07:04
 0:02:01
0:02:01
 0:12:05
0:12:05
 0:03:33
0:03:33
 0:12:30
0:12:30
 0:06:33
0:06:33
 0:07:14
0:07:14
 1:03:46
1:03:46
 0:11:00
0:11:00
 0:17:15
0:17:15
 0:27:57
0:27:57
 0:07:46
0:07:46
 0:09:03
0:09:03
 0:00:59
0:00:59
 0:01:03
0:01:03