filmov
tv
Thermodynamics - Entropy 7.1 Clausius Inequality

Показать описание
Thermodynamics - Clausius Inequality
Like and subscribe! And get the notes here:
Like and subscribe! And get the notes here:
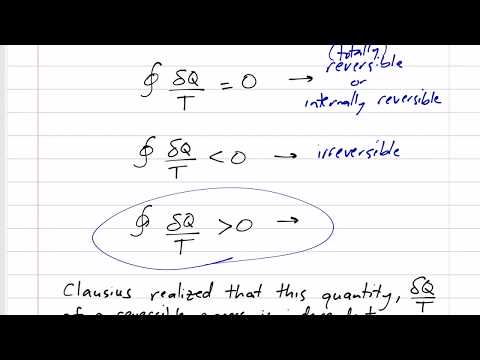
Thermodynamics - Entropy 7.1 Clausius Inequality
Thermodynamics - ENTROPY as a Property in 12 Minutes!
Thermo 07 Entropy 1 Clausius
Thermo 7.1: Entropy and Clausius Inequality
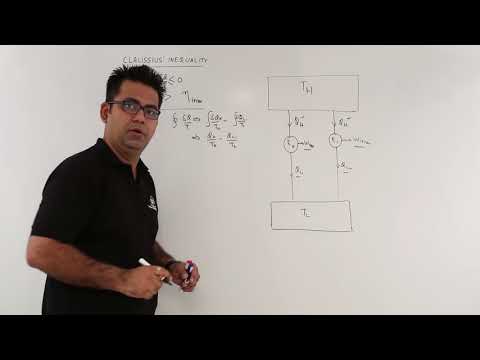
Clausius Inequality
Thermodynamics Lecture 16: Clausius Inequality
ENGR251: We needed an inequality: Clausius inequality
What is entropy? - Jeff Phillips
Entropy Definiton, Clausius Inequality statemnt, conditions of reversible, irreversible, impossible
MET 320 Entropy The Clausius Statement
Clausius Theorem
Thermodynamics-Clausius Inequality- Engineering Chemistry 1 Notes (CY6151)
Clausius Inequality and Entropy
Equivalence of Kelvin Planck and Clausius Statements | Mechanical Engineering |Benchmark Engineering
The Second Law of Thermodynamics: Entropy, Clausius & Kelvin Plank Statements
The Clausius-Duhem Inequality — Lesson 5, Part 1
Chapter 7: Clausius/Clapeyron Example 1
Thermodynamics Lecture 17: Entropy
Clausius Inequality (Important), Part-1, Entropy, Thermodynamics, GATE Mechanical Online Lectures
Class 11 chapter 6 | Thermodynamics 10 | What is ENTROPY ? | Spontaneity and Entropy JEE MAINS /NEET
What is Entropy? | Clausius Inequality - Physics with GeeklyEDU
Thermo 07 Entropy 2 Entropy Clausius
Thermodynamics I - The Clausius Inequality
2nd Law of thermodynamics - Principles of Refrigeration
Комментарии
 0:13:12
0:13:12
 0:11:59
0:11:59
 0:13:31
0:13:31
 0:16:02
0:16:02
 0:07:46
0:07:46
 0:02:59
0:02:59
 0:13:33
0:13:33
 0:05:20
0:05:20
 0:20:50
0:20:50
 0:27:34
0:27:34
 0:09:05
0:09:05
 0:05:27
0:05:27
 0:07:03
0:07:03
 0:03:39
0:03:39
 0:08:22
0:08:22
 0:22:57
0:22:57
 0:15:18
0:15:18
 0:10:06
0:10:06
 0:21:39
0:21:39
 0:56:21
0:56:21
 0:11:20
0:11:20
 0:20:55
0:20:55
 0:07:41
0:07:41
 0:07:41
0:07:41