filmov
tv
Clausius Inequality

Показать описание
Clausius Inequality
Lecture By: Er. Himanshu Vasishta, Tutorials Point India Private Limited
Lecture By: Er. Himanshu Vasishta, Tutorials Point India Private Limited
Thermodynamics - Entropy 7.1 Clausius Inequality
Clausius Inequality
ENGR251: We needed an inequality: Clausius inequality
The Clausius Inequality | Physical Chemistry I | 042
Understanding Entropy Change and Clausius Inequality.
Clausius inequality | Thermodynamic world view, chapter 19
Example: Applying the Clausius inequality to a cycle
Thermodynamics - ENTROPY as a Property in 12 Minutes!
Second law of thermodynamics: Clausius's inequality
Clausius Inequality Example 1
Thermodynamics Lecture 16: Clausius Inequality
V3A 4 Clausius Inequality
7.1 | Clausius Inequality | Prof Uddipta Ghosh | ES-211 Thermodynamics
ENGR251: Clausius inequality: is my cat a genius?
Clausius Inequality (Lecture 3.12)
3.11 Clausius Inequality
7.1 Introducing Clausius Inequality
Thermodynamics-Clausius Inequality- Engineering Chemistry 1 Notes (CY6151)
Clausius Theorem
Thermodynamics lecture 8 - Clausius inequality derivation and proof
Clausius Inequality and Entropy
Clausius inequality, application of second law
Thermodynamics I - The Clausius Inequality
Solved examples on Clausius inequality & change in entropy - GATE 2021 examination
Комментарии
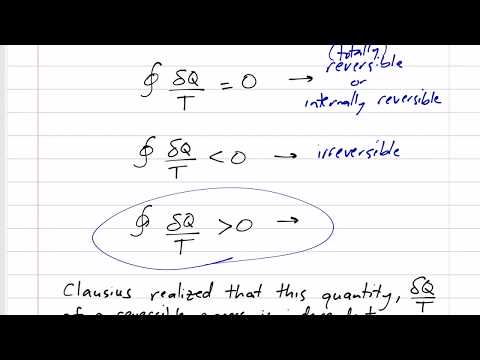 0:13:12
0:13:12
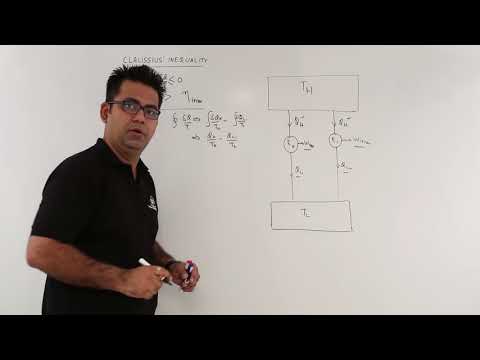 0:07:46
0:07:46
 0:13:33
0:13:33
 0:08:31
0:08:31
 0:18:01
0:18:01
 0:06:44
0:06:44
 0:05:26
0:05:26
 0:11:59
0:11:59
 0:18:35
0:18:35
 0:04:42
0:04:42
 0:02:59
0:02:59
 0:06:59
0:06:59
 0:23:03
0:23:03
 0:10:12
0:10:12
 0:02:59
0:02:59
 0:30:20
0:30:20
 0:12:17
0:12:17
 0:05:27
0:05:27
 0:15:58
0:15:58
 0:19:03
0:19:03
 0:07:03
0:07:03
 0:20:33
0:20:33
 0:07:41
0:07:41
 0:05:39
0:05:39