filmov
tv
Kinetic Molecular Theory of Gases - Practice Problems

Показать описание
This chemistry video tutorial explains the concept of the kinetic molecular theory of gases. It contains a few multiple choice practice problems as well. The assumptions of this model states that the volume of gas particles are negligible. Gas molecules do not exert forces on each other. They neither attract or repel. Gas particles are in constant random motion and the average kinetic energy of a gas sample is directly proportional to the kelvin temperature. The kinetic molecular theory describes the behavior of ideal gases. real gases that are nonpolar tend to behave more like ideal gases than polar gases. This video explains the concept of common gas laws such as boyle's law, charles law, gay lussac's law, and avogadro's law all in one video. Other topics including in this video are pressure, volume, temperature, moles, gas density, root mean square velocity, average kinetic energy and molar mass of gases.
Gas Laws Formula Sheet:
Pressure & Boiling Point:
Gas Pressure Unit Conversion:
Manometers & Barometers:
Water Height & Mercury Column:
Boyle's Law Practice Problems:
_________________________________
How Does a Bike Pump Work?
Charles Law:
Gay Lussac's Law:
Avogadro's Law:
Ideal Gas Law Problems:
Combined Gas Law Problems:
_______________________________
Gas Stoichiometry Problems:
Molar Mass of a Gas at STP:
Gas Density at STP:
Dalton's Law of Partial Pressure:
Collecting Gas Over Water:
_______________________________
Gas Density of Mixtures:
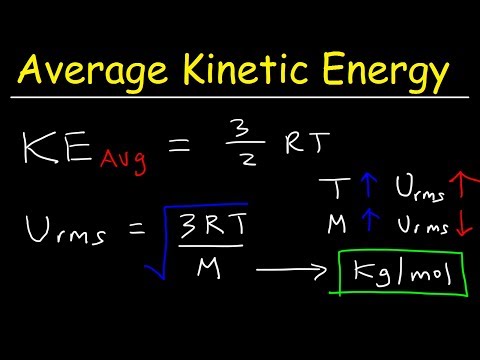
Average Kinetic Energy of a Gas:
Graham's Law of Effusion:
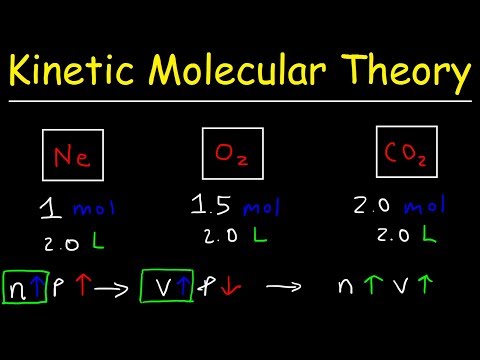
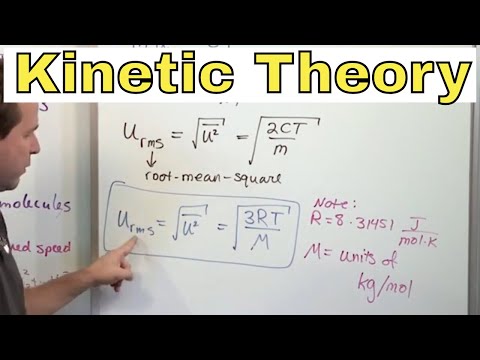
Kinetic Molecular Theory of Gases:
Gas Law Problems Review:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Gas Laws Formula Sheet:
Pressure & Boiling Point:
Gas Pressure Unit Conversion:
Manometers & Barometers:
Water Height & Mercury Column:
Boyle's Law Practice Problems:
_________________________________
How Does a Bike Pump Work?
Charles Law:
Gay Lussac's Law:
Avogadro's Law:
Ideal Gas Law Problems:
Combined Gas Law Problems:
_______________________________
Gas Stoichiometry Problems:
Molar Mass of a Gas at STP:
Gas Density at STP:
Dalton's Law of Partial Pressure:
Collecting Gas Over Water:
_______________________________
Gas Density of Mixtures:
Average Kinetic Energy of a Gas:
Graham's Law of Effusion:
Kinetic Molecular Theory of Gases:
Gas Law Problems Review:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:05:11
0:05:11
 0:06:24
0:06:24
 0:07:00
0:07:00
 0:08:10
0:08:10
 0:43:21
0:43:21
 0:14:56
0:14:56
 0:01:31
0:01:31
 0:15:00
0:15:00
 0:29:48
0:29:48
 0:07:40
0:07:40
 0:09:09
0:09:09
 0:11:28
0:11:28
 0:04:13
0:04:13
 0:05:58
0:05:58
 0:08:11
0:08:11
 0:04:34
0:04:34
 0:20:35
0:20:35
 0:28:05
0:28:05
 0:12:51
0:12:51
 0:21:29
0:21:29
 0:18:37
0:18:37
 0:19:24
0:19:24
 0:11:25
0:11:25
 0:09:05
0:09:05