filmov
tv
Chemical Reactions (4 of 11) Decomposition Reactions, An Explanation

Показать описание
Describes the basics of decomposition reactions, how to identify them, predict the products and balance the chemical equation. Two examples are also shown, decomposition of sugar and hydrogen peroxide.
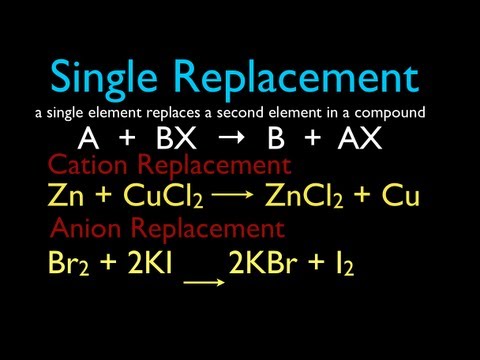
A chemical reaction is a process that leads to the chemical change of one set of chemical substances to another. Chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms. In a chemical reaction there is no change to the nuclei of the atoms. They can often be described by a chemical equation. Chemical equations are used to graphically illustrate chemical reactions. They consist of the chemical formulas of the of the reactants on the left and those of the products on the right. They are separated by an arrow (→) which indicates the direction and type of the reaction. The most common types of chemical reactions are: double replacement, single replacement, combustion, decomposition and synthesis.
Also, please don't forget to do all of the following;
(1) Subscribe to my channel, Step-By-Step Science.
(2) Give me a thumbs up for this video.
(3) Leave me a nice positive comment.
(4) Sharing is Caring! Share this video with others in need.
Thank you! I greatly appreciate all of your support.
A chemical reaction is a process that leads to the chemical change of one set of chemical substances to another. Chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms. In a chemical reaction there is no change to the nuclei of the atoms. They can often be described by a chemical equation. Chemical equations are used to graphically illustrate chemical reactions. They consist of the chemical formulas of the of the reactants on the left and those of the products on the right. They are separated by an arrow (→) which indicates the direction and type of the reaction. The most common types of chemical reactions are: double replacement, single replacement, combustion, decomposition and synthesis.
Also, please don't forget to do all of the following;
(1) Subscribe to my channel, Step-By-Step Science.
(2) Give me a thumbs up for this video.
(3) Leave me a nice positive comment.
(4) Sharing is Caring! Share this video with others in need.
Thank you! I greatly appreciate all of your support.
Комментарии
 0:08:14
0:08:14
 0:12:54
0:12:54
 0:00:19
0:00:19
 0:40:06
0:40:06
 0:05:18
0:05:18
 0:18:42
0:18:42
 0:06:50
0:06:50
 0:18:49
0:18:49
 0:00:47
0:00:47
 0:04:17
0:04:17
 0:20:53
0:20:53
 0:03:46
0:03:46
 0:10:13
0:10:13
 0:14:56
0:14:56
 0:03:00
0:03:00
 0:01:01
0:01:01
 0:05:03
0:05:03
 0:04:50
0:04:50
 0:09:28
0:09:28
 0:05:21
0:05:21
 0:08:06
0:08:06
 0:05:01
0:05:01
 0:00:23
0:00:23
 0:13:05
0:13:05