filmov
tv
Types of Chemical Reactions

Показать описание
Types of Chemical Reactions: Combination, Decomposition, Displacement, Double Displacement and Redox reactions are discussed in this video. The chemical reactions are visualized in an interesting way and many examples are shown.
Special Offer on our Full Courses!
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Special Offer on our Full Courses!
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Types of Chemical Reactions
Types of Chemical Reactions
Types of Chemical Reactions
Types of Chemical Reactions
Types of Chemical Reactions
Classifying Types of Chemical Reactions Practice Problems
Types of Chemical Reactions
The 5 Different Types of Chemical Reactions
NDA Online Classes | Free Complete Course of Chemistry | Topic : Types of Chemical Reactions
Every Type Of Chemical Reaction Explained in 9 Minutes
Types of Chemical Reactions
Types of Chemical Reactions
TYPES OF CHEMICAL REACTIONS | Animation
Chemical Reactions Types - Single vs Double displacement, Combination, Decomposition & Combustio...
Types of Chemical Reactions: Study Hall Chemistry #2: ASU + Crash Course
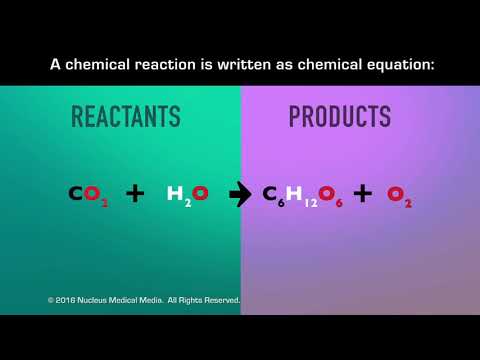
5 Types of Chemical Reactions (Chemistry) + Activity Series, Solubility Rules
Learn the 6 Types of Chemical Reactions (Chemistry)
Chemical Reactions
Classifying Types of Chemical Reactions With Practice Problems | Study Chemistry With Us
All You Need To Know About Types of Chemical Reactions | Explained in 17min for GED Science
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
Types Of Chemical Reactions - Synthesis Reactions, Decomposition Reactions, And Exchange Reactions
What triggers a chemical reaction? - Kareem Jarrah
Types of Chemical Reactions| Anatomy and Physiology
Комментарии
 0:12:54
0:12:54
 0:40:06
0:40:06
 0:05:10
0:05:10
 0:03:00
0:03:00
 0:20:53
0:20:53
 0:05:48
0:05:48
 0:03:31
0:03:31
 0:03:05
0:03:05
 0:52:56
0:52:56
 0:09:34
0:09:34
 0:05:34
0:05:34
 0:08:06
0:08:06
 0:04:30
0:04:30
 0:13:31
0:13:31
 0:11:41
0:11:41
 0:15:01
0:15:01
 0:06:05
0:06:05
 0:04:21
0:04:21
 0:17:50
0:17:50
 0:17:31
0:17:31
 0:18:42
0:18:42
 0:02:58
0:02:58
 0:03:46
0:03:46
 0:09:02
0:09:02