filmov
tv
Structure of Atom | Atomic Structure | One Shot | #BounceBack Series | Unacademy Atoms | Sakshi Vora

Показать описание
00:00 Introduction
6:52 Dalton's atomic theory
12:00 Thomson model
14:20 discovery of electron
24:23 discovery of proton
38:17 Rutherford model of atom
50:28 coulomb law
57:32 waves
1:07:30 EM waves
1:13:05 EM spectrum
1:16:05 Plancks quantum theory
1:30:06 photoelectric effect
1:56:06 spectrum
2:00:18 Bohr's atomic theory
3:14:11 absorption and emission energy
3:26:53 line spectrum of hydrogen
3:45:18 de-broglie hypothesis
4:00:05 Heisenberg's uncertainty
4:11:00 Electronic Configuration
4:13:00 Wave mechanical model
5:18:57 quantum number
5:51:05 important graphs , please do it
6:09:49 electronic configuration.
#UnacademyAtoms #BounceBack #StructureOfAtom #SakshiVora
GCSE Physics - Atomic Structure, Isotopes & Electrons Shells #32
Chemistry - Atomic Structure - EXPLAINED!
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons
Atom Explained in Simple Terms
Basic Atomic Structure: A Look Inside the Atom
What is an atom | Matter | Physics | FuseSchool
The 2,400-year search for the atom - Theresa Doud
GCSE Chemistry - Atoms & Ions #1
Chemistry 11 - Atomic Structure - Part 3
What Are The Different Atomic Models? Dalton, Rutherford, Bohr and Heisenberg Models Explained
GCSE Chemistry - History of the Model of the Atom #7
What's Inside an Atom? Protons, Electrons, and Neutrons!
The Basic Structure of the Atom | Chemistry and Our Universe: How it All Works
Structure of an Atom
Atomic Structure: Protons, Electrons & Neutrons | Chemistry
Atomic Structure: Protons, Electrons & Neutrons
Structure of the atom
Atom Structure | Matter | Physics | FuseSchool
What Is An Atom? | The Dr. Binocs Show | Best Learning Videos For Kids | Peekaboo Kidz
What is an Atom ?
structure of atom class 11 all formulas
A Brief History Of Atom | Democritus to Quantum | Atomic Models
What is an Atom? - Structure of an Atom - Atom video for kids
The History of Atomic Chemistry: Crash Course Chemistry #37
Комментарии
 0:05:22
0:05:22
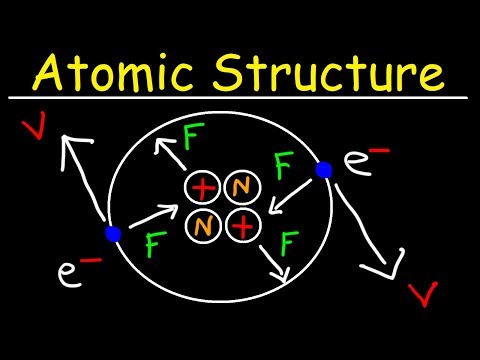 0:11:45
0:11:45
 0:02:20
0:02:20
 0:01:44
0:01:44
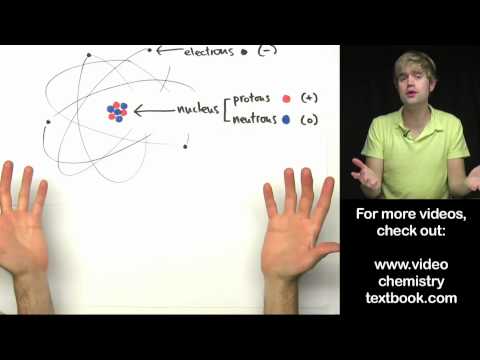 0:07:44
0:07:44
 0:04:00
0:04:00
 0:05:23
0:05:23
 0:07:20
0:07:20
 1:04:37
1:04:37
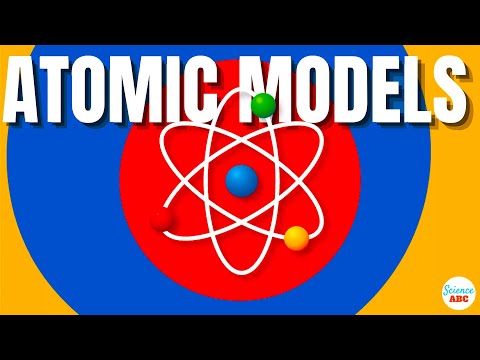 0:07:04
0:07:04
 0:04:32
0:04:32
 0:04:06
0:04:06
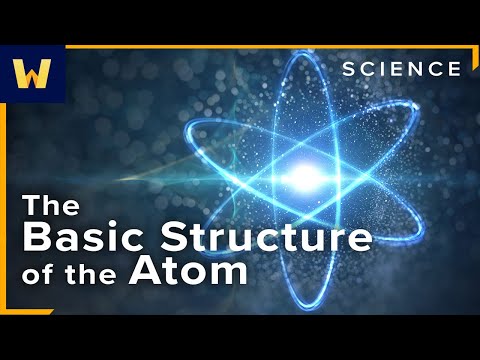 0:30:31
0:30:31
 0:02:02
0:02:02
 0:07:02
0:07:02
 0:13:31
0:13:31
 0:05:16
0:05:16
 0:04:38
0:04:38
 0:07:17
0:07:17
 0:09:49
0:09:49
 0:00:12
0:00:12
 0:33:07
0:33:07
 0:03:26
0:03:26
 0:09:42
0:09:42