filmov
tv
How much heat will be required to make `2kg` of calcium carbide `(CaC_(2))` according to the

Показать описание
How much heat will be required to make `2kg` of calcium carbide `(CaC_(2))` according to the following reaction? `CaO(s) +3C(s) rarr CaC_(2)(s) +CO(g)` The heats of formations of `caO(s), CaC_(2)(s)`, and `CO(g)` are `-151.0,-14.0`,and `-26.0 kcal`, respectively.
 0:04:16
0:04:16
 0:04:13
0:04:13
 0:04:19
0:04:19
 0:02:16
0:02:16
 0:05:33
0:05:33
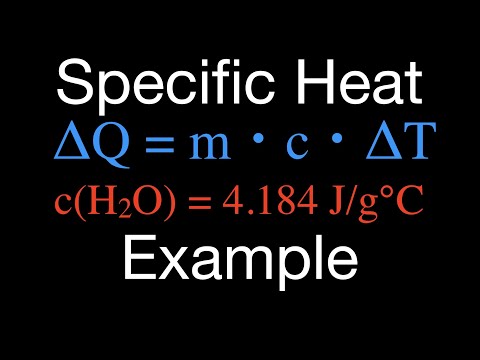 0:04:38
0:04:38
 0:10:46
0:10:46
 0:02:16
0:02:16
 0:01:44
0:01:44
 0:09:50
0:09:50
 0:01:43
0:01:43
 0:03:15
0:03:15
 0:11:09
0:11:09
 0:06:46
0:06:46
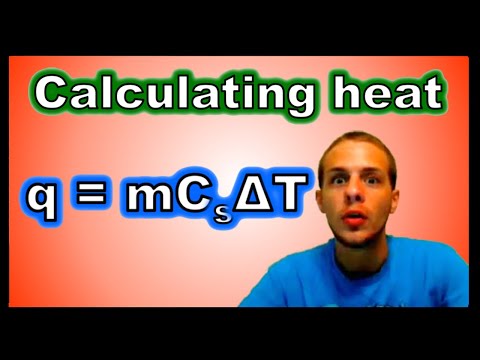 0:07:05
0:07:05
 0:02:05
0:02:05
 0:02:38
0:02:38
 0:03:52
0:03:52
 0:01:40
0:01:40
 0:11:41
0:11:41
 0:07:18
0:07:18
 0:03:53
0:03:53
 0:06:48
0:06:48
 0:22:38
0:22:38