filmov
tv
Writing Ionic and Covalent Formulas

Показать описание
Writing Ionic and Covalent Formulas. Mr. Causey shows how easy it is to write ionic compound and covalent compound formulas. All you need is a periodic table, a list of polyatomic ions, and some simple rules and you're ready to start writing formulas.
SUBSCRIBE for more chemistry videos:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra, and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos to choose from.
CONTACT ME:
FOLLOW ME:
RESOURCES
Polyatomic Ion Cheat Sheet:
Periodic Table:
RELATED VIDEOS:
Writing Ionic Formulas:
Writing Covalent Formulas:
Naming Compounds:
Naming Ionic Compounds:
Naming Covalent Compounds:
In this lesson, we'll go through the formula anatomy, binary compounds, writing formulas, and ionic and covalent formulas. You will need a periodic table unless you have it memorized. You will need a list of the major polyatomic ions.
And if you get my list, you'll have a list of the metal cations and the numeric prefixes as well. You need to know oxidation numbers, polyatomic ions, the periodic table, covalent and ionic bonds, and the chemical names. It is very important to know these things. Especially, be familiar with the covalent and ionic bonds. So, a chemical formula, what is it? It's a symbolic representation of a chemical compound. For instance, potassium permanganate, its symbolic representation would be KMnO4.
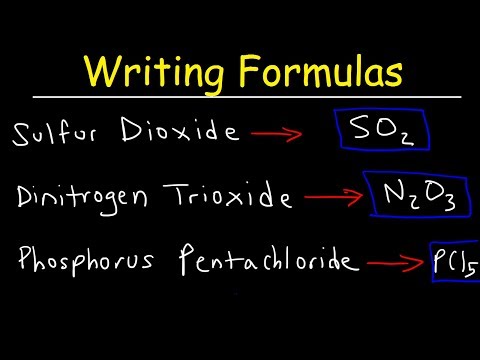
Let's take a look at formula anatomy. You need to make sure you understand how formulas are put together. Here's the formula for "calcium fluoride". The "Ca" and the "F", or the calcium and the fluorine, that's the elements. The small numbers below and to the right are called subscripts. Subscripts tell you how many atoms or ions are needed in that substance. There must be two fluorine ions in calcium fluoride. So, we put a two for the subscript. Since there is only one calcium ion, we don't write the one, similar to algebra.
The number out in front is the coefficient and tells you how many formula units, molecules, or moles are being used. Remember, most compounds are binary, meaning they have two parts. KCl or H2O. Notice that KCl is potassium and chlorine and H2O is hydrogen and oxygen.
#mrcausey #yourchemcoach #chemicalformulas
SUBSCRIBE for more chemistry videos:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra, and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos to choose from.
CONTACT ME:
FOLLOW ME:
RESOURCES
Polyatomic Ion Cheat Sheet:
Periodic Table:
RELATED VIDEOS:
Writing Ionic Formulas:
Writing Covalent Formulas:
Naming Compounds:
Naming Ionic Compounds:
Naming Covalent Compounds:
In this lesson, we'll go through the formula anatomy, binary compounds, writing formulas, and ionic and covalent formulas. You will need a periodic table unless you have it memorized. You will need a list of the major polyatomic ions.
And if you get my list, you'll have a list of the metal cations and the numeric prefixes as well. You need to know oxidation numbers, polyatomic ions, the periodic table, covalent and ionic bonds, and the chemical names. It is very important to know these things. Especially, be familiar with the covalent and ionic bonds. So, a chemical formula, what is it? It's a symbolic representation of a chemical compound. For instance, potassium permanganate, its symbolic representation would be KMnO4.
Let's take a look at formula anatomy. You need to make sure you understand how formulas are put together. Here's the formula for "calcium fluoride". The "Ca" and the "F", or the calcium and the fluorine, that's the elements. The small numbers below and to the right are called subscripts. Subscripts tell you how many atoms or ions are needed in that substance. There must be two fluorine ions in calcium fluoride. So, we put a two for the subscript. Since there is only one calcium ion, we don't write the one, similar to algebra.
The number out in front is the coefficient and tells you how many formula units, molecules, or moles are being used. Remember, most compounds are binary, meaning they have two parts. KCl or H2O. Notice that KCl is potassium and chlorine and H2O is hydrogen and oxygen.
#mrcausey #yourchemcoach #chemicalformulas
Комментарии
 0:07:14
0:07:14
 0:11:01
0:11:01
 0:10:22
0:10:22
 0:02:53
0:02:53
 0:11:44
0:11:44
 0:10:32
0:10:32
 0:04:17
0:04:17
 0:05:44
0:05:44
 0:17:56
0:17:56
 0:13:33
0:13:33
 0:10:46
0:10:46
 0:06:26
0:06:26
 0:59:54
0:59:54
 0:10:11
0:10:11
 0:21:57
0:21:57
 0:11:25
0:11:25
 0:03:42
0:03:42
 0:11:58
0:11:58
 0:10:41
0:10:41
 0:08:43
0:08:43
 0:07:21
0:07:21
 0:39:25
0:39:25
 0:11:21
0:11:21
 0:01:30
0:01:30