filmov
tv
Electron Configuration Ruthenium (Electron configuration exceptions)
Показать описание
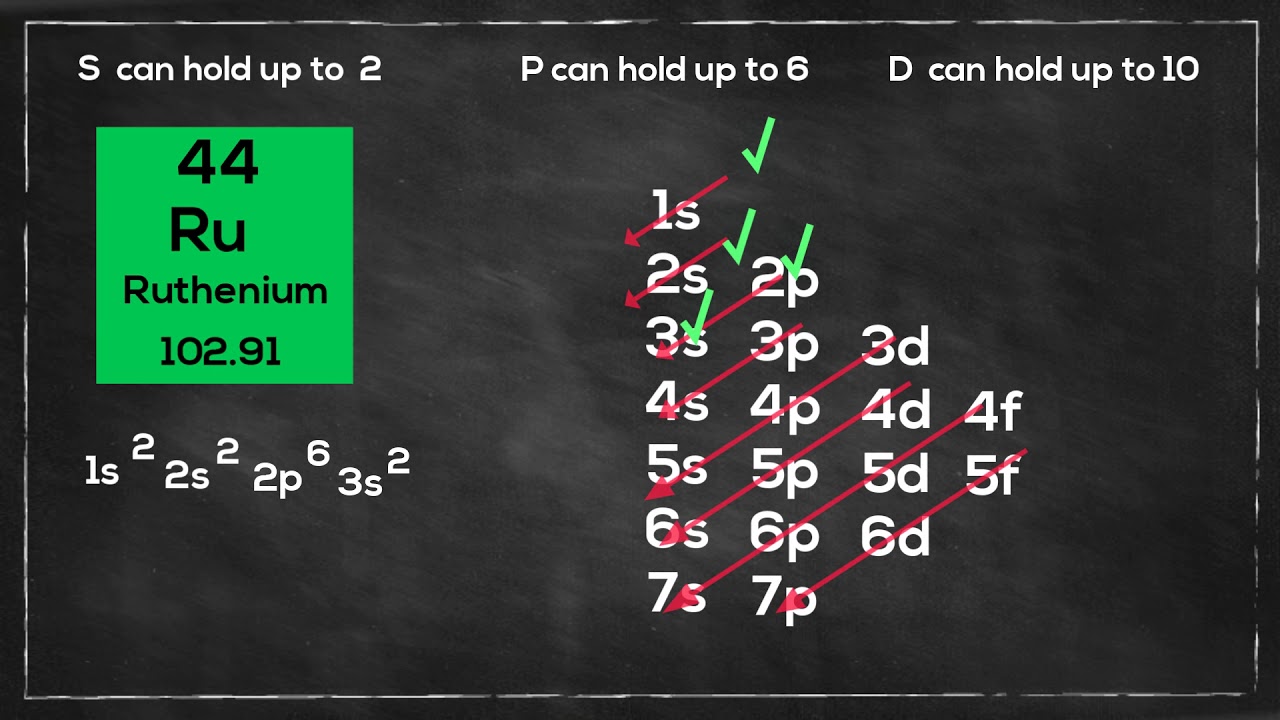
Electron configuration Ruthenium
Ruthenium has an atomic number of 44 and has 44 electrons.
It is an exception to the normal rules of electron configuration because instead of having filled the 5s orbital with 2 electrons and the 4d orbital with 6, Ruthenium fills the 5s orbital with 2 electrons and the 4d orbital with 7 electrons.
Ruthenium has an atomic number of 44 and has 44 electrons.
It is an exception to the normal rules of electron configuration because instead of having filled the 5s orbital with 2 electrons and the 4d orbital with 6, Ruthenium fills the 5s orbital with 2 electrons and the 4d orbital with 7 electrons.
Electron Configuration Ruthenium (Electron configuration exceptions)
Electron Configuration of Ruthenium Ru Lesson
Exceptional Electron Configuration of Ruthenium | Inorganic Chemistry
Full and Abbreviated Electron Configuration of Ruthenium Ru
Ruthenium (Ru) symbol chemical element of the periodic table
Electron configurations with the periodic table | Chemistry | Khan Academy
Electron Configuration - Rhodium
Electronic configuration of Ruthenium is an exception!
exceptional electronic configuration of Ruthenium (Ru) part 4
Introduction to electron configurations | AP Chemistry | Khan Academy
6.5 Electron Configuration | General Chemistry
Electron Configuration Exceptions Examples: Cr, Cu, Ag, and Mo
How to write electron configurations and what they are
electron configuration big element
Electron Configuration: The Easy Way and Without Memorizing Anything
Electronic Configuration Best Trick 🥰🥰
Electron Configurations
Electronic configuration of first 54 elements (atoms)
How to Write the Electron Configuration of an Element | Study Chemistry With Us
Electron Configuration Practice - Ir
Electron Configuration Rhodium (Exception to the Rule)
Electron configurations of ions | Atomic structure and properties | AP Chemistry | Khan Academy
Electron Configuration Exceptions
Full and Abbreviated Electron Configuration of Rhodium Rh
Комментарии
 0:01:48
0:01:48
 0:05:24
0:05:24
 0:06:49
0:06:49
 0:00:28
0:00:28
 0:00:41
0:00:41
 0:14:39
0:14:39
 0:02:03
0:02:03
 0:00:30
0:00:30
 0:00:51
0:00:51
 0:05:08
0:05:08
 0:44:18
0:44:18
 0:02:59
0:02:59
 0:17:24
0:17:24
 0:02:26
0:02:26
 0:09:21
0:09:21
 0:00:12
0:00:12
 0:09:31
0:09:31
 0:31:15
0:31:15
 0:30:45
0:30:45
 0:03:27
0:03:27
 0:01:45
0:01:45
 0:03:56
0:03:56
 0:04:30
0:04:30
 0:00:28
0:00:28