filmov
tv
Recrystallization

Показать описание
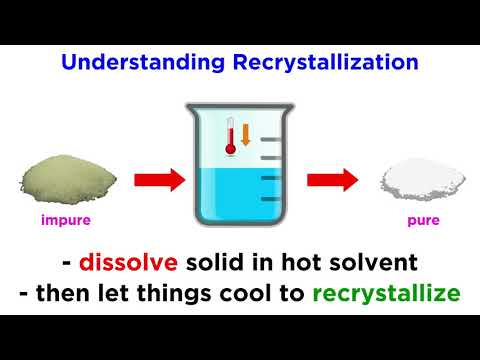
Now that we have covered a variety of separation techniques, we know how to get an isolated product! But if it's a solid, it may contain impurities. Recrystallization is a common organic chemistry laboratory technique for the purification of solids, so let's learn how to do it!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Recrystallization
Recrystallization and Melting Point Analysis
Recrystallization
Introduction to Recrystallization
Technique Series: Recrystallization (urea as an example)
Recovery, Recyrstallization, and Grain Growth
How to Purify by Recrystallization
How To Recrystallize A Solid
How to make Potassium Nitrate with household chemicals
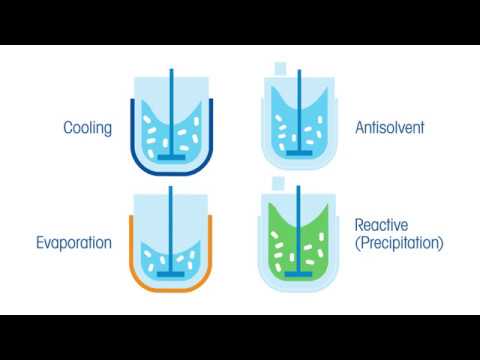
4 Recrystallization Methods for Increased Yield
[Orgo Lab] Recrystallization of Acetanilide
Recrystallization of Amines: A Step-by-Step Purification Guide
Recrystallisation
Recrystallization
Recrystallization
Recrystallization : Practical
Quick Revision - Recrystallisation
Recrystallization - Performing the Technique
Recrystallization to Increase the Purity.
Purification of Benzoic Acid by Crystallization - MeitY OLabs
Recrystallization
How to Perform a Recrystallization
Disclination mediated dynamic recrystallization in metals at low temperature
A short recrystallization demonstration (phthalic acid)
Комментарии
 0:05:51
0:05:51
 0:11:04
0:11:04
 0:01:15
0:01:15
 0:07:15
0:07:15
 0:18:21
0:18:21
 0:07:34
0:07:34
 0:05:07
0:05:07
 0:05:41
0:05:41
 0:03:49
0:03:49
 0:03:21
0:03:21
![[Orgo Lab] Recrystallization](https://i.ytimg.com/vi/mfI0OZjbHQc/hqdefault.jpg) 0:03:38
0:03:38
 0:03:59
0:03:59
 0:11:22
0:11:22
 0:20:12
0:20:12
 0:04:48
0:04:48
 0:05:51
0:05:51
 0:02:29
0:02:29
 0:11:45
0:11:45
 0:01:17
0:01:17
 0:03:42
0:03:42
 0:34:19
0:34:19
 0:03:37
0:03:37
 0:00:23
0:00:23
 0:01:30
0:01:30