filmov
tv
How to Purify by Recrystallization

Показать описание
We show how to purify aluminum nitrate and strontium nitrate by recrystallization. This is important because those two substances must be extremely pure for our upcoming video on making glow in the dark powder.
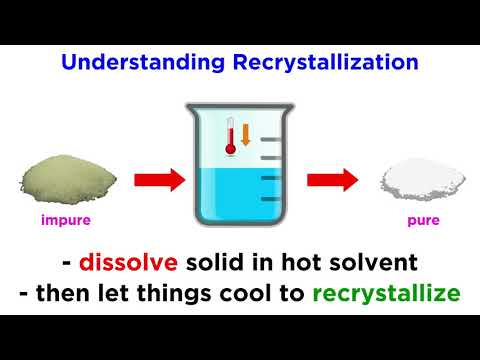
Dissolve the dry aluminum nitrate in water and filter off any insoluble materials. We could not filter it off before it dried in the previous video because the particles at that stage are much too small. It needs crystallize once to aggregate into particles large that can be filtered.
After drying, carefully weigh out the crystals. Then take that mass and add in 20% mass of water. So if you have 50g like me, you add 10g of water (or 10mL since density is 1g/mL).
It won't all dissolve, so carefully heat the mixture until it dissolves. Then cover it and let it cool down. Eventually it'll crystallize out purer crystals.
After the mixture cools to room temperature, and left for a few hours. The liquid is poured off and discarded, it has the impurities and is not needed.
The crystals are again weighed and once again they are recrystallized with 20% water.
Once this is done. Dry the final product. I started with 50g and ended up with 17g. But now it's very pure. Better than 99%
Strontium nitrate also needs to be purified. But it's solubility doesn't change that much with temperature so we'll have to purify by slow evaporation recrystallization rather than thermal cycle recrystallization.
Once again, dissolve it in water and filter it. Then let it crystallize. But before it dries completely, let the liquid reduce to about 20% of it's saturated volume. So I started with a saturated solution of 10mL of strontium nitrate, i then waited until it evaporated down to 2mL of fluid. The crystals are not included in this assessment. Then discard the liquid. Repeat the recrystallization process.
Once again, use the desiccator bag to obtain extremely dry chemicals.
We are now ready to produce glow-in-the-dark powder.
Dissolve the dry aluminum nitrate in water and filter off any insoluble materials. We could not filter it off before it dried in the previous video because the particles at that stage are much too small. It needs crystallize once to aggregate into particles large that can be filtered.
After drying, carefully weigh out the crystals. Then take that mass and add in 20% mass of water. So if you have 50g like me, you add 10g of water (or 10mL since density is 1g/mL).
It won't all dissolve, so carefully heat the mixture until it dissolves. Then cover it and let it cool down. Eventually it'll crystallize out purer crystals.
After the mixture cools to room temperature, and left for a few hours. The liquid is poured off and discarded, it has the impurities and is not needed.
The crystals are again weighed and once again they are recrystallized with 20% water.
Once this is done. Dry the final product. I started with 50g and ended up with 17g. But now it's very pure. Better than 99%
Strontium nitrate also needs to be purified. But it's solubility doesn't change that much with temperature so we'll have to purify by slow evaporation recrystallization rather than thermal cycle recrystallization.
Once again, dissolve it in water and filter it. Then let it crystallize. But before it dries completely, let the liquid reduce to about 20% of it's saturated volume. So I started with a saturated solution of 10mL of strontium nitrate, i then waited until it evaporated down to 2mL of fluid. The crystals are not included in this assessment. Then discard the liquid. Repeat the recrystallization process.
Once again, use the desiccator bag to obtain extremely dry chemicals.
We are now ready to produce glow-in-the-dark powder.
Комментарии
 0:05:07
0:05:07
 0:05:51
0:05:51
 0:09:42
0:09:42
 0:11:04
0:11:04
 0:03:42
0:03:42
 0:07:17
0:07:17
 0:08:21
0:08:21
 0:04:24
0:04:24
 0:18:21
0:18:21
 0:02:04
0:02:04
 0:11:08
0:11:08
 0:11:45
0:11:45
 0:00:57
0:00:57
 0:06:00
0:06:00
 0:04:30
0:04:30
 0:05:41
0:05:41
 0:03:20
0:03:20
 0:00:44
0:00:44
 0:20:12
0:20:12
 0:01:01
0:01:01
 0:07:25
0:07:25
![[Orgo Lab] Recrystallization](https://i.ytimg.com/vi/mfI0OZjbHQc/hqdefault.jpg) 0:03:38
0:03:38
 0:03:45
0:03:45
 0:10:14
0:10:14