filmov
tv
Ligand Field Theory and the Jahn-Teller Effect

Показать описание
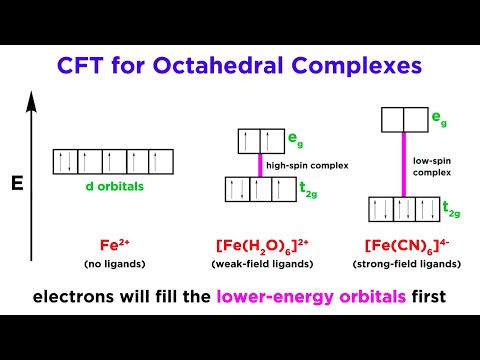
We've learned about a number of theories regarding chemical bonding, like VSEPR Theory, Molecular Orbital Theory, and Crystal Field Theory. Now let's look at Ligand Field Theory, which is sort of an extension of CFT. We will also examine the Jahn-Teller Effect, or Jahn-Teller Distortion, which is a phenomenon that impacts the geometry of certain complexes.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Ligand Field Theory and the Jahn-Teller Effect
Ligand Field Theory and Spectrochemical Series | Professor Adam Teaches
Crystal Field Theory
Crystal Field Theory
Ligand Field Theory: Understanding Coordination Complex Electronic Structures!
Ligand Field Theory/Molecular orbital Theory for Coordination Compounds sigma and pi bonding
Ligand Field Theory of Tetrahedral Complexes
Ligand Field Theory
Pi Bonding Ligands | Pi Donor and Pi Acceptors Ligands | Ligand Field Theory | MOT Diagram
Ligand Field Theory & Bond Strength – The Power of Sigma & Pi Interactions
481 - 10 Ligand Field Theory
21.4 Crystal Field Theory | General Chemistry
28. Transition Metals: Crystal Field Theory Part I
Ligand Field Theory Basics 1: SALCs of Sigma-Only Donors, Pi-Donors, and Pi-Acceptors in Oh Symmetry
Ligand field theory Meaning
Crystal Field Theory | Easy Trick
Crystal Field Theory ; Octahedral Splitting
Ligand Field Theory Basics 2: Ligand Field Diagrams in Oh Symmetry and the Spectrochemical Series
crystal field splitting in tetrahedral complexes #ytshorts #youtubeshorts
Ligand Field Theory Basics 3: 4-Coordinate Geometries
Ligand Field Theory - Mo Diagram For Sigma Bonding
in the octahedral ligand field theory the 3d orbitals will split into
Ligand Field Theory: An Oh complex
Crystal Field Theory (Octahedral Geometry) for Coordination Compounds
Комментарии
 0:07:45
0:07:45
 0:15:53
0:15:53
 0:07:42
0:07:42
 0:21:56
0:21:56
 0:24:59
0:24:59
 0:38:55
0:38:55
 0:08:55
0:08:55
 0:20:31
0:20:31
 0:19:21
0:19:21
 0:16:21
0:16:21
 0:12:55
0:12:55
 0:23:25
0:23:25
 0:53:35
0:53:35
 0:23:13
0:23:13
 0:00:35
0:00:35
 0:15:32
0:15:32
 0:01:11
0:01:11
 0:24:47
0:24:47
 0:00:31
0:00:31
 0:30:29
0:30:29
 0:09:43
0:09:43
 0:00:35
0:00:35
 0:25:34
0:25:34
 0:18:16
0:18:16