filmov
tv
STRUCTURE OF ATOM in 1 Shot || FULL Chapter Coverage (Concepts+PYQs) || Class 11th Chemistry

Показать описание
-------------------------------------------------------
Dive deep into the heart of "Structure of Atom" with this one-shot wonder! 🧪 Class 11th Chemistry enthusiasts, explore the entire chapter with comprehensive coverage of concepts and previous year questions. Elevate your understanding and ensure success in exams. Watch now for a condensed journey through atomic complexities!
-------------------------------------------------------.
PACE SERIES CLASS 11th Details
✒️ This batch is completely FREE for all the students aiming Class-11th CBSE Students.
✒️ We will cover the CBSE Board Syllabus of Physics, Chemistry, Biology, Maths & English.
✒️ We will cover each chapter in One Shot with PYQs.
✒️ India’s Best Faculty Team will teach in this batch.
✒️ RECORDED Lecture will be provided on the NCERT WALLAH Youtube Channel.
✒️ Lecture Notes will be provided on our PW App.
✒️ Classes will start from 25th December 2023.
✒️ Most Important Questions Practice Sheet will be provided with each Chapter Completion.
✒️ Weekly lecture schedule will be provided on the NCERT WALLAH Community Section.
✒️ Classes will be held on Weekly basis from Monday to Saturday.
✒️ 2 Classes will be provided Daily at 3pm & 6pm.
-------------------------------------------------------
📌 𝐍𝐂𝐄𝐑𝐓 𝐖𝐀𝐋𝐋𝐀𝐇 𝐒𝐎𝐂𝐈𝐀𝐋 𝐌𝐄𝐃𝐈𝐀 -
-------------------------------------------------------
📌 𝐏𝐇𝐘𝐒𝐈𝐂𝐒 𝐖𝐀𝐋𝐋𝐀𝐇 𝐒𝐎𝐂𝐈𝐀𝐋 𝐌𝐄𝐃𝐈𝐀 -
-------------------------------------------------------
⏳Timestamps:
00:00 - Introduction
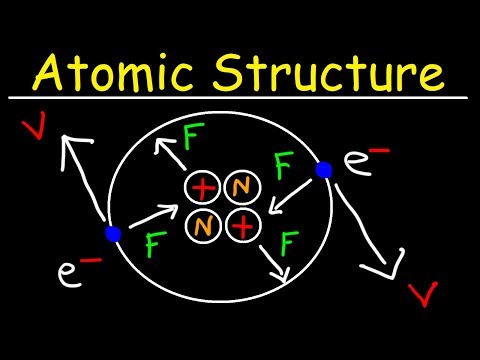
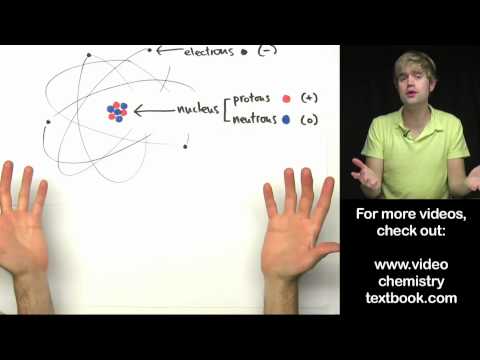
04:11 - Atom
07:12 - Discovery of electron
28:26 - Discovery of anode rays
39:48 - Discovery of neutron
43:08 - Representation of atom
47:27 - Isotopes and isobars
51:55 - Atomic models
1:13:27 - Electromagnetic wave theory
1:35:29 - Electromagnetic spectrum
1:43:06 - Planks quantum theory
1:59:24 - Photoelectric effect
2:20:26 - Bohr’s model postulates
2:42:33 - Spectrum
2:56:38 - de-Broglie concept
3:03:08 - Heisenberg uncertainty principle
3:15:10 - Quantum number
3:38:32 - Bohr’s bury rule and Aufbau principle
3:57:20 - Thank You Bacchon!
-------------------------------------------------------
-------------------------------------------------------
#StructureOfAtom #Class11 #NCERTWallah #PhysicsWallah #PaceSeriesClass11 #Chemistry #Concepts #PYQs #OneShot #Class11Chemistry #Atom
Комментарии
 0:11:45
0:11:45
 0:02:20
0:02:20
 1:57:06
1:57:06
 0:00:12
0:00:12
 3:11:55
3:11:55
 0:07:44
0:07:44
 2:32:00
2:32:00
 7:19:15
7:19:15
 0:23:24
0:23:24
 1:18:31
1:18:31
 0:01:44
0:01:44
 5:13:38
5:13:38
 1:12:03
1:12:03
 0:09:49
0:09:49
 0:09:49
0:09:49
 1:41:32
1:41:32
 0:10:43
0:10:43
 1:04:43
1:04:43
 0:10:12
0:10:12
 0:00:27
0:00:27
 0:00:35
0:00:35
 1:50:52
1:50:52
 0:59:50
0:59:50
 0:04:26
0:04:26