filmov
tv
Why Can't a Strong Acid Be Used to Make a Buffer?

Показать описание
This video discusses the reasons why strong acid (or strong bases) cannot be used to make a buffer solution.
Why Can't a Strong Acid Be Used to Make a Buffer?
How to Determine if Acid is Strong or Weak Shortcut w/ Examples and Practice Problems
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton
How To Memorize The Strong Acids | Trick for Strong Acid & Weak Acid #shorts #reels #jee
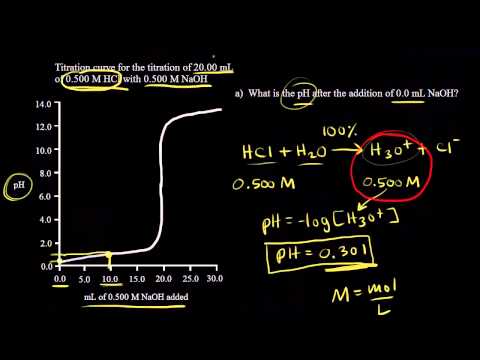
Titration of Strong Acid With Strong Base
What is a Buffer?
Identifying the acid and base in an acid-base reaction #acidsandbases #organicchemistry
General Chemistry | Strong Acid and Base Calculations
ACID AND BASES (QUESTION 7): PHYSICAL SCIENCES P2 NOV 2023 (GR 11)
Acid Base Strength - Which Is Stronger?
The Strongest Acids in the World
Effect of adding Water on the pH of the Strong Acid
What is an acid-base buffer?
Strong acid–strong base reactions | Acids and bases | AP Chemistry | Khan Academy
Weak base–strong acid reactions | Acids and bases | AP Chemistry | Khan Academy
Strong Acid | Strong Base | Tricks to Learn Strong Acid & Strong Base | Chemistry By Kajal Ma&ap...
pH of Strong Acids | A-Level Chemistry
Must Strong Acid be pH 1
Why HCL acid in Stomach Doesn't Damage Stomach | Why acid Doesn't Harm Stomach
Strong and Weak Acids
General Chemistry | Strong Acid & Base Titration
Titration of a strong acid with a strong base | Chemistry | Khan Academy
Acids and Bases - Reaction with each other | Don't Memorise
Strong acid solutions | Acids and bases | AP Chemistry | Khan Academy
Комментарии
 0:02:35
0:02:35
 0:02:34
0:02:34
 0:03:48
0:03:48
 0:00:49
0:00:49
 0:08:27
0:08:27
 0:04:27
0:04:27
 0:00:34
0:00:34
 0:15:35
0:15:35
 0:19:43
0:19:43
 0:11:39
0:11:39
 0:04:49
0:04:49
 0:03:11
0:03:11
 0:01:01
0:01:01
 0:09:29
0:09:29
 0:06:28
0:06:28
 0:01:47
0:01:47
 0:08:00
0:08:00
 0:05:14
0:05:14
 0:03:46
0:03:46
 0:08:10
0:08:10
 0:18:51
0:18:51
 0:10:12
0:10:12
 0:02:22
0:02:22
 0:06:18
0:06:18