filmov
tv
SN1 or SN2? Explanation in description! | Organic Chemistry

Показать описание
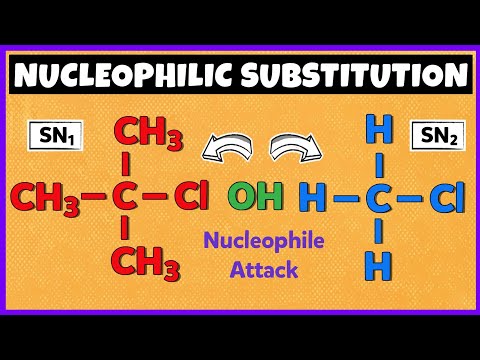
Can you tell if this is more likely to undergo SN1 or SN2 type reaction mechanism?
Explanation:
- An important factor in this decision is the type of carbon that the leaving group is attached to.
- The leaving group is the chlorine, and the carbon that the chlorine is attached to is a tertiary carbon (it has three alkyl or CH groups attached to it). This is important to note for two reasons:
i) In an SN1 reaction, the first step is that the leaving group pops off, forming a carbocation. Carbocations are unstable and will generally only form if stabilized via resonance or inductive effects. Therefore, this reaction can only be SN1 if there is something to stabilize the carbocation. For this starting molecule, there are three electron-donating alkyl groups attached to the carbon that can stabilize a carbocation that will form in an SN1 reaction mechanism. This points towards an SN1 type reaction mechanism.
ii) In an SN2 reaction, the nucleophile attacks, and the leaving group leaves in the same step. For this to happen, the nucleophile needs space to be able to attack the atom attached to the leaving group. For this starting molecule, the three alkyl groups attached to carbon of the leaving group introduce steric hindrance that prevent the nucleophile from attacking at the same time. This suggests that an SN2 type reaction mechanism will not occur.
Together, these two points suggest an SN1 type reaction mechanism!
#organicchemistry #shorts #chemistry organic chemistry, organic one, orgo one, Sn2, Sn1, reaction mechanisms, predicting reaction mechanisms
Explanation:
- An important factor in this decision is the type of carbon that the leaving group is attached to.
- The leaving group is the chlorine, and the carbon that the chlorine is attached to is a tertiary carbon (it has three alkyl or CH groups attached to it). This is important to note for two reasons:
i) In an SN1 reaction, the first step is that the leaving group pops off, forming a carbocation. Carbocations are unstable and will generally only form if stabilized via resonance or inductive effects. Therefore, this reaction can only be SN1 if there is something to stabilize the carbocation. For this starting molecule, there are three electron-donating alkyl groups attached to the carbon that can stabilize a carbocation that will form in an SN1 reaction mechanism. This points towards an SN1 type reaction mechanism.
ii) In an SN2 reaction, the nucleophile attacks, and the leaving group leaves in the same step. For this to happen, the nucleophile needs space to be able to attack the atom attached to the leaving group. For this starting molecule, the three alkyl groups attached to carbon of the leaving group introduce steric hindrance that prevent the nucleophile from attacking at the same time. This suggests that an SN2 type reaction mechanism will not occur.
Together, these two points suggest an SN1 type reaction mechanism!
#organicchemistry #shorts #chemistry organic chemistry, organic one, orgo one, Sn2, Sn1, reaction mechanisms, predicting reaction mechanisms
 0:18:52
0:18:52
 0:12:19
0:12:19
 0:08:03
0:08:03
 0:13:31
0:13:31
 0:07:29
0:07:29
 0:03:30
0:03:30
 0:00:59
0:00:59
 0:15:16
0:15:16
 0:38:50
0:38:50
 0:00:47
0:00:47
 0:05:16
0:05:16
 0:06:05
0:06:05
 0:00:05
0:00:05
 0:00:44
0:00:44
 0:02:00
0:02:00
 1:13:40
1:13:40
 0:22:49
0:22:49
 0:00:53
0:00:53
 0:26:25
0:26:25
 0:00:11
0:00:11
 0:34:46
0:34:46
 0:08:45
0:08:45
 0:00:52
0:00:52
 0:18:28
0:18:28