filmov
tv
Sulfur Electron Configuration
Показать описание
A step-by-step description of how to write the electron configuration for Sulfur (S).
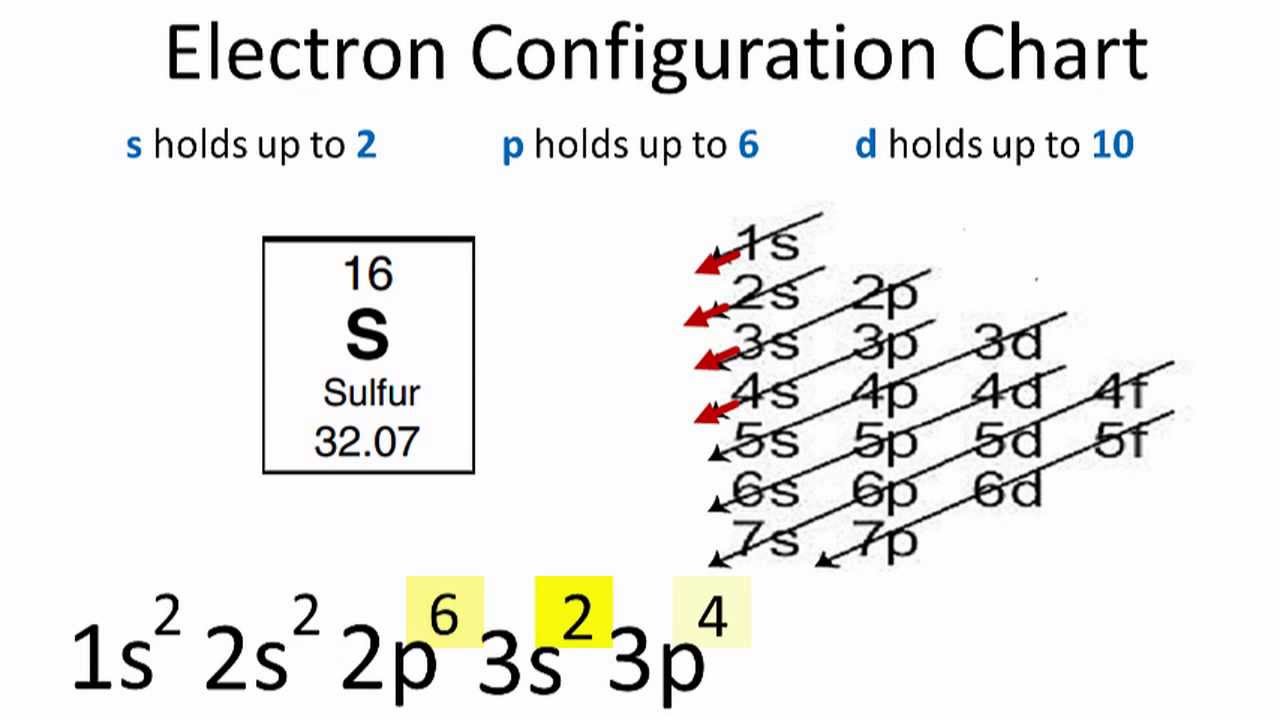
In order to write the S electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16electrons in orbitals around the nucleus of the Sulfur atom.
In this video we'll use the electron configuration chart to help us write the notation for Sulfur. Note that the last term in the Sulfur electron configuration will be 1s2 2s2 2p6 3s2 3p4.
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
In order to write the S electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16electrons in orbitals around the nucleus of the Sulfur atom.
In this video we'll use the electron configuration chart to help us write the notation for Sulfur. Note that the last term in the Sulfur electron configuration will be 1s2 2s2 2p6 3s2 3p4.
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Sulfur Electron Configuration
Sulphur Electron Configuration
Sulfur Electron Configuration | How to Write the Electron Configuration for Sulfur
Electron Configuration - Basic introduction
Electron Configuration Phosphorus and Sulfur
9 what is the electron configuration of sulfur??
Sulfur electronic configuration,how to Write Sulfur electronic configuration
How to Write the Electron Configuration for an Element in Each Block
electronic configuration for S(sulfur).. #k2chemistryclass #electronicconfiguration
Electron Configuration of Sulfur S Lesson
sulfur electron configuration
Electron Configuration for Sulfur (S) 16🤗 FASTEST ANSWER to your Homework
Sulfur Ground State Electron Configuration
S 2- Electron Configuration (Sulfide Ion)
Electron Configuration Orbital Diagram Sulfur
Full and Abbreviated Electron Configuration of Sulfur S
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Electronic Configuration Of Sulphur || S Electronic Configuration || 😱😱🔥🔥#shorts #viralshorts...
Atomic Structure (Bohr Model) for Sulfur (S)
💯 √ Electron Configuration of Carbon, Sulfur and Calcium Atoms
Electronic configuration Shell and subshell of sulphur #mswalishwetamam #shortvideo #youtubeshorts
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
How to draw Electron-in-box diagrams Electronic Configurations? [GCE A Level Chemistry]
Electron Configuration With Noble Gas Notation
Комментарии
 0:01:38
0:01:38
 0:00:59
0:00:59
 0:01:55
0:01:55
 0:10:19
0:10:19
 0:02:21
0:02:21
 0:00:53
0:00:53
 0:01:47
0:01:47
 0:07:23
0:07:23
 0:00:57
0:00:57
 0:04:44
0:04:44
 0:03:25
0:03:25
 0:00:45
0:00:45
 0:03:28
0:03:28
 0:02:46
0:02:46
 0:02:53
0:02:53
 0:00:28
0:00:28
 0:01:00
0:01:00
 0:00:58
0:00:58
 0:02:05
0:02:05
 0:02:32
0:02:32
 0:00:19
0:00:19
 0:12:12
0:12:12
 0:04:28
0:04:28
 0:10:06
0:10:06