filmov
tv
Sulphur Electron Configuration

Показать описание
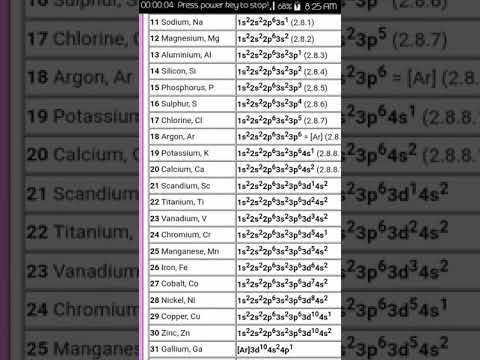
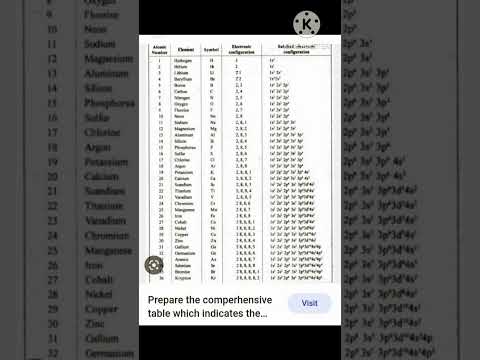
Sulphur has 16 electrons. The electron configuration is 2, 8, 8. Sulfur is a non-metal that is pale yellow, odorless and brittle. Sulfur is used in the vulcanisation of black rubber, as a fungicide and in black gunpowder. Most sulfur is, however, used in the production of sulfuric acid, which is perhaps the most important chemical manufactured by western civilisations. The most important of sulfuric acid’s many uses is in the manufacture of phosphoric acid, to make phosphates for fertilisers.
Sulfur occurs naturally as the element, often in volcanic areas. This has traditionally been a major source for human use. It is also widely found in many minerals including iron pyrites, galena, gypsum and Epsom salts.
Sulfur is essential to all living things. It is taken up as sulfate from the soil (or seawater) by plants and algae. It is used to make two of the essential amino acids needed to make proteins. It is also needed in some co-enzymes. The average human contains 140 grams and takes in about 1 gram a day, mainly in proteins.
Sulfur and sulfate are non-toxic. However, carbon disulfide, hydrogen sulfide and sulfur dioxide are all toxic. Hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis.
Sulfur dioxide is produced when coal and unpurified oil are burned. Sulfur dioxide in the atmosphere causes acid rain. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things.
Sulfur occurs naturally as the element, often in volcanic areas. This has traditionally been a major source for human use. It is also widely found in many minerals including iron pyrites, galena, gypsum and Epsom salts.
Sulfur is essential to all living things. It is taken up as sulfate from the soil (or seawater) by plants and algae. It is used to make two of the essential amino acids needed to make proteins. It is also needed in some co-enzymes. The average human contains 140 grams and takes in about 1 gram a day, mainly in proteins.
Sulfur and sulfate are non-toxic. However, carbon disulfide, hydrogen sulfide and sulfur dioxide are all toxic. Hydrogen sulfide is particularly dangerous and can cause death by respiratory paralysis.
Sulfur dioxide is produced when coal and unpurified oil are burned. Sulfur dioxide in the atmosphere causes acid rain. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things.
 0:01:38
0:01:38
 0:00:59
0:00:59
 0:00:57
0:00:57
 0:01:47
0:01:47
 0:02:21
0:02:21
 0:02:11
0:02:11
 0:00:16
0:00:16
 0:02:18
0:02:18
 0:02:46
0:02:46
 0:00:45
0:00:45
 0:02:03
0:02:03
 0:05:48
0:05:48
 0:02:05
0:02:05
 0:02:32
0:02:32
 0:00:19
0:00:19
 0:02:53
0:02:53
 0:12:12
0:12:12
 0:00:58
0:00:58
 0:01:15
0:01:15
 0:00:12
0:00:12
 0:01:01
0:01:01
 0:13:36
0:13:36
 0:00:05
0:00:05
 0:01:48
0:01:48