filmov
tv
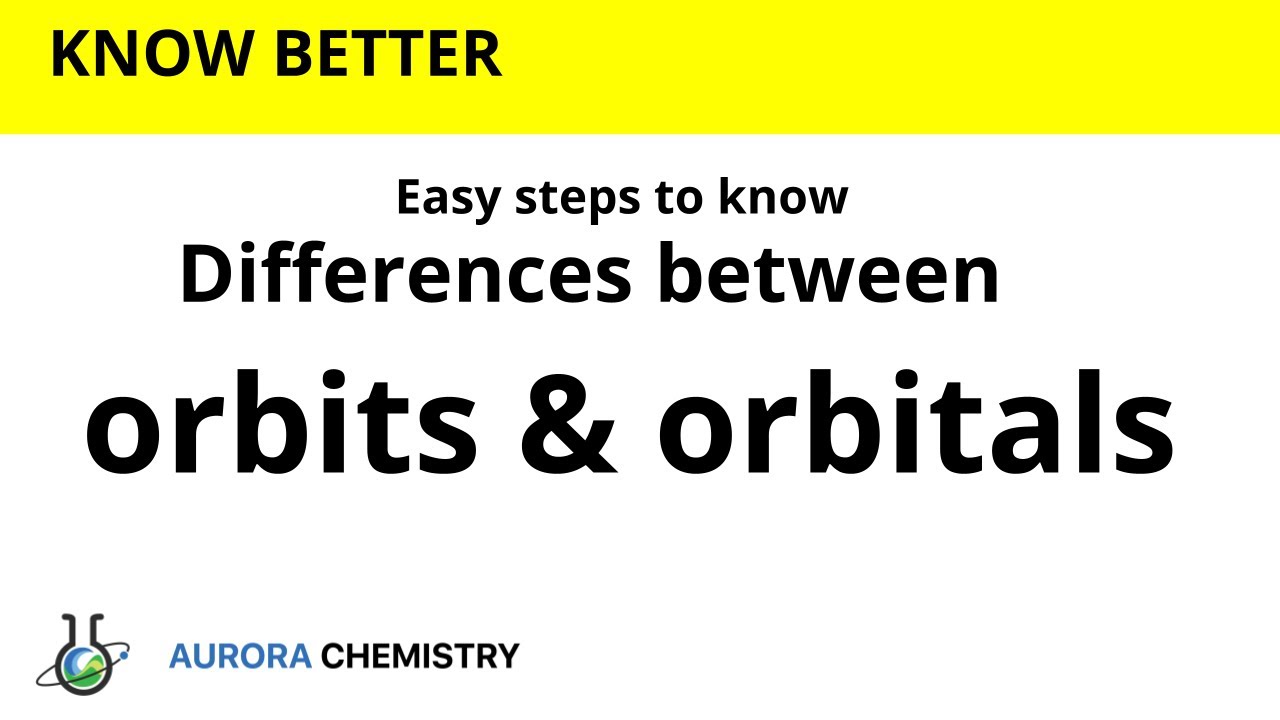
What are the differences between ORBITS and ORBITALS ?
Показать описание
This video states differences between orbit and orbital.
Orbit:
1. It is a circular path around nucleus where electrons are revolving.
2. It represents 2D motion of electron
3. The maximum number of electrons in orbit is 2n2, Here n stands for number of orbit.
4. Since orbits are non-directional, they cannot explain shape of molecules.
Orbitals:
1. It is a region of space around the nucleus where probability of finding electron is higher..
2. It represents 3D motion of electron.
3.The maximum number of electrons in each orbital is 2
4.Orbitals are directional and hence they account for shapes of molecules.
Orbit:
1. It is a circular path around nucleus where electrons are revolving.
2. It represents 2D motion of electron
3. The maximum number of electrons in orbit is 2n2, Here n stands for number of orbit.
4. Since orbits are non-directional, they cannot explain shape of molecules.
Orbitals:
1. It is a region of space around the nucleus where probability of finding electron is higher..
2. It represents 3D motion of electron.
3.The maximum number of electrons in each orbital is 2
4.Orbitals are directional and hence they account for shapes of molecules.
 0:11:41
0:11:41
 0:31:06
0:31:06
 0:00:51
0:00:51
 0:19:44
0:19:44
 0:04:25
0:04:25
 0:07:14
0:07:14
 0:09:17
0:09:17
 0:05:32
0:05:32
 0:00:41
0:00:41
 0:04:50
0:04:50
 0:09:54
0:09:54
 0:05:31
0:05:31
 0:06:58
0:06:58
 0:01:22
0:01:22
 0:00:06
0:00:06
 0:02:06
0:02:06
 0:57:12
0:57:12
 0:00:56
0:00:56
 0:08:18
0:08:18
 0:00:47
0:00:47
 0:04:39
0:04:39
 0:11:01
0:11:01
 0:05:56
0:05:56
 0:04:47
0:04:47