filmov
tv
Dilutions Made Easy--How to create solutions of any concentration whatsoever! By Dr. Shawn

Показать описание
The ability to create solutions with particular concentrations is a foundational skill for the chemical and biological sciences. In this video, Dr. Shawn* shows you the practical secrets to simply and easily create chemical solutions with any concentration(s) you need. Intended for chemistry students and citizen scientists.
(OOPS: Apologies for the error at 7:33. The top box should read "39% solvent", not "31%".)
Measuring Density:
SUPPLIES USED IN THIS VIDEO:
Volumetric Flasks
Inexpensive Digital Scale
Volumetric Pipettes
(OOPS: Apologies for the error at 7:33. The top box should read "39% solvent", not "31%".)
Measuring Density:
SUPPLIES USED IN THIS VIDEO:
Volumetric Flasks
Inexpensive Digital Scale
Volumetric Pipettes
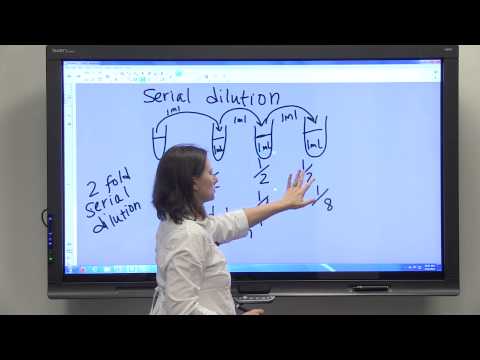
How to prepare a Serial Dilution
Dilutions Made Easy--How to create solutions of any concentration whatsoever! By Dr. Shawn
Dilutions- An Introduction
1:20 Dilution.Two easy methods to prepare.learn & understand then can use other methods (In Engl...
How to Dilute a Solution
Determining the Total Dilution Factor
How to Make a Serial Dilution
Dilutions in clinical chemistry: the math in detail
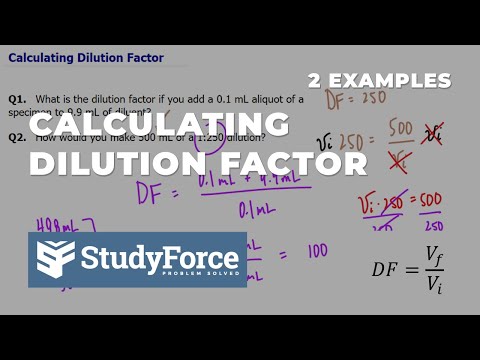
Calculating Dilution Factor
Dilutions Made Easy
How to do Dilution and Serial Dilution? | Dilution Factor Calculations
How to Prepare 0.1 M NaOH Solution?|| Calculations and Experiment
Simple Dilutions
Serial Dilution | Required Practical Revision for Biology and Chemistry A-Level
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Preparation of various dilutions from stock/standard solution.
Dilution Calculations for Neurotoxins - Finally Made Easy!
How to make dilutions for perfumery (VISUAL DEMONSTRATION)
Dilution Series & Serial Dilution
Serial dilution calculations | How to calculate dilution factor - Dr K
Chemistry Made Easy: Dilutions
1:10 Dilution formula | How to make 1:10 Dilution
How to Perform Serial Dilutions in Microbiology
How to Use the Dilution Equation
Комментарии
 0:03:16
0:03:16
 0:09:00
0:09:00
 0:05:44
0:05:44
 0:03:46
0:03:46
 0:03:36
0:03:36
 0:07:14
0:07:14
 0:07:03
0:07:03
 0:02:04
0:02:04
 0:02:33
0:02:33
 0:02:44
0:02:44
 0:13:25
0:13:25
 0:04:11
0:04:11
 0:04:19
0:04:19
 0:04:24
0:04:24
 0:08:46
0:08:46
 0:06:34
0:06:34
 0:04:00
0:04:00
 0:07:03
0:07:03
 0:08:32
0:08:32
 0:05:05
0:05:05
 0:09:34
0:09:34
 0:01:52
0:01:52
 0:06:13
0:06:13
 0:10:35
0:10:35