filmov
tv
Determining the Total Dilution Factor

Показать описание
Determining the Total Dilution Factor
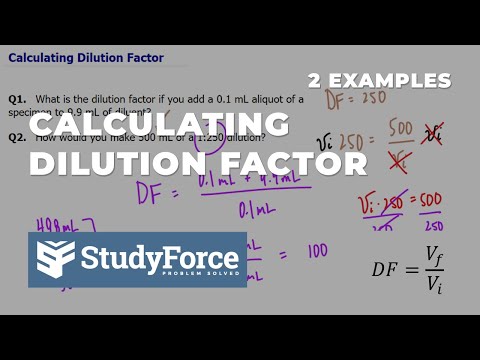
Calculating Dilution Factor
Serial dilution calculations | How to calculate dilution factor - Dr K
How to prepare a Serial Dilution
How to Calculate Dilution Factor
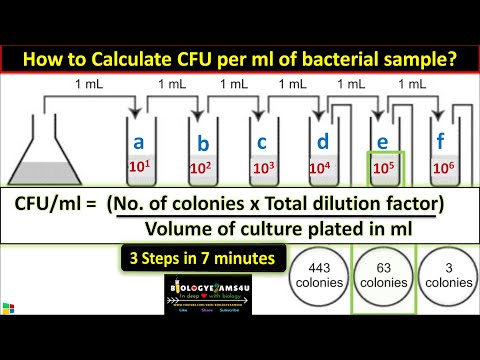
How to Calculate CFU per ml of Bacterial Sample? in 3 Steps || cfu/ml in Microbiology
Dilution and Dilution Factor in Microbiology|| How to Calculate Dilution factor in Serial dilution?
Finding the dilution factor
Dilution factor
1:20 Dilution.Two easy methods to prepare.learn & understand then can use other methods (In Engl...
Serial Dilution Calculation
Dilutions- An Introduction
How Do You Calculate Dilution Factor?
CPP#20 - How to calculate dilution factor? - Dr K
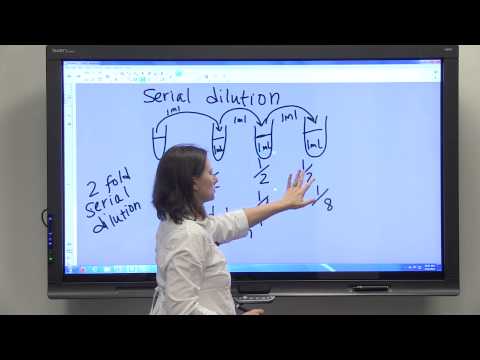
Dilution Series & Serial Dilution
Calculating the concentration of a solution using a dilution factor
CPP#21: Serial dilution calculations | Calculate final concentration - Dr K
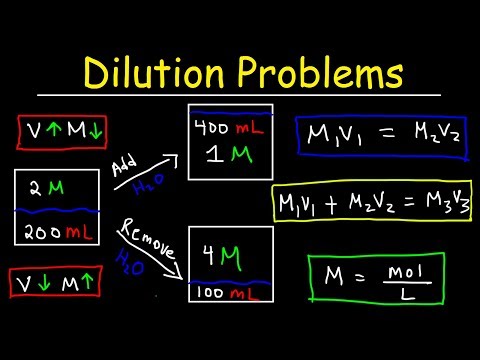
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
How to do Dilution and Serial Dilution? | Dilution Factor Calculations
AS Biology - How to calculate serial and simple dilutions
what is the dilution factor? and how to calculate it? #pharma #dilution #pharmacy
Dilutions in clinical chemistry: the math in detail
Serial Dilutions | Microbiology
How to Perform Serial Dilution for Bacterial Growth Measurement: Step-by-Step Guide
Комментарии
 0:07:14
0:07:14
 0:02:33
0:02:33
 0:05:05
0:05:05
 0:03:16
0:03:16
 0:01:16
0:01:16
 0:07:07
0:07:07
 0:05:07
0:05:07
 0:04:05
0:04:05
 0:01:48
0:01:48
 0:03:46
0:03:46
 0:02:38
0:02:38
 0:05:44
0:05:44
 0:03:09
0:03:09
 0:02:04
0:02:04
 0:08:32
0:08:32
 0:04:40
0:04:40
 0:04:24
0:04:24
 0:21:55
0:21:55
 0:13:25
0:13:25
 0:09:16
0:09:16
 0:01:00
0:01:00
 0:02:03
0:02:03
 0:10:03
0:10:03
 0:03:01
0:03:01