filmov
tv
Determining a Rate LAW from experimental data: Result is 1st order

Показать описание
Multiple experiments are run, varying the initial concentrations and measuring the rate. From the multiple experiments, we can solve for the exponents, and then the rate constant, k. this ends up being 1st order. 1st order equations are derived in this video.
How to Find the Rate Law and Rate Constant (k)
Kinetics: Initial Rates and Integrated Rate Laws
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
Reaction Order Tricks & How to Quickly Find the Rate Law
Worked example: Determining a rate law using initial rates data | AP Chemistry | Khan Academy
The Rate Law
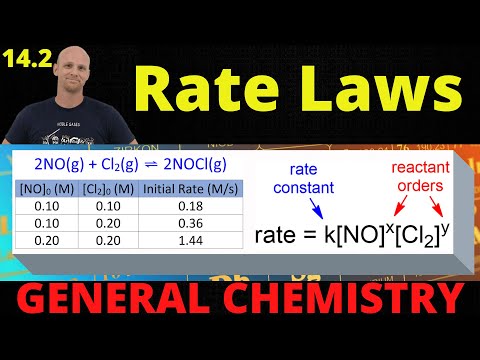
14.2 Rate Laws | General Chemistry
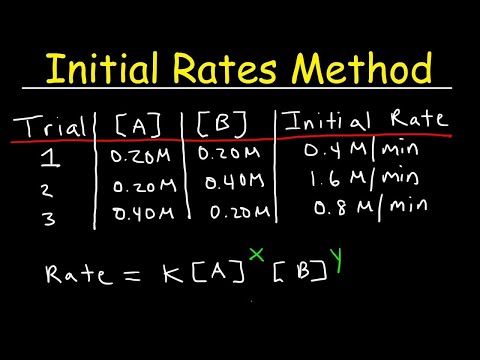
Chemical Kinetics - Initial Rates Method
Determining Rate Law Form
Experimental method to determine the rate law: E2
Solving a Rate Law Using the Initial Rates Method
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
Determine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I
Determining Rate Law
Determining Reaction Order From Rate Law 001
Week 5 - 1. Determining a rate law
Determination of rate law and rate expression from experimental data
Determining the Rate Law for a Mechanism with a Slow First Step- Example
DON'T MISS THIS Rate Law and Rate Constant Question
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
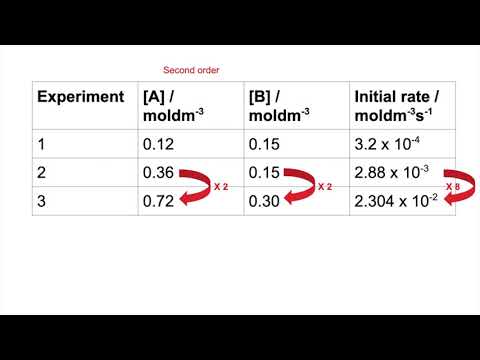
Working out order from a rate table - tricky example
Concentration-Time Data - Determination of Rate Law and Rate Constant
Rate Determining Step
How to Determine the Form of the Rate Law | Professor Dave & Chegg Explain
Комментарии
 0:03:42
0:03:42
 0:09:10
0:09:10
 0:18:48
0:18:48
 0:01:58
0:01:58
 0:12:28
0:12:28
 0:08:44
0:08:44
 0:25:16
0:25:16
 0:34:53
0:34:53
 0:09:36
0:09:36
 0:04:58
0:04:58
 0:10:49
0:10:49
 0:48:46
0:48:46
 0:07:46
0:07:46
 0:12:35
0:12:35
 0:02:43
0:02:43
 0:08:17
0:08:17
 0:09:00
0:09:00
 0:02:15
0:02:15
 0:03:46
0:03:46
 0:08:42
0:08:42
 0:02:46
0:02:46
 0:05:41
0:05:41
 0:00:24
0:00:24
 0:04:21
0:04:21