filmov
tv
Gas Density and Molar Mass Problems at STP - Chemistry

Показать описание
This chemistry video tutorial explains how to calculate gas density given the molar mass at STP. It also explains how gas density relates to pressure, temperature and molar mass.
Pressure & Boiling Point:
Gas Pressure Unit Conversion:
Manometers & Barometers:
Water Height & Mercury Column:
Boyle's Law Practice Problems:
_________________________________
How Does a Bike Pump Work?
Charles Law:
Gay Lussac's Law:
Avogadro's Law:
Ideal Gas Law Problems:
Combined Gas Law Problems:
_______________________________
Gas Stoichiometry Problems:
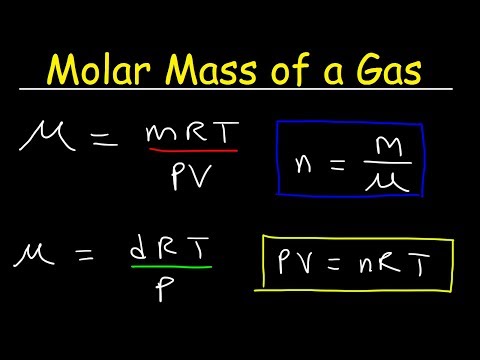
Molar Mass of a Gas at STP:
Gas Density at STP:
Dalton's Law of Partial Pressure:
Collecting Gas Over Water:
_______________________________
Gas Density of Mixtures:
Average Kinetic Energy of a Gas:
Graham's Law of Effusion:
Kinetic Molecular Theory of Gases:
Gas Law Problems Review:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Pressure & Boiling Point:
Gas Pressure Unit Conversion:
Manometers & Barometers:
Water Height & Mercury Column:
Boyle's Law Practice Problems:
_________________________________
How Does a Bike Pump Work?
Charles Law:
Gay Lussac's Law:
Avogadro's Law:
Ideal Gas Law Problems:
Combined Gas Law Problems:
_______________________________
Gas Stoichiometry Problems:
Molar Mass of a Gas at STP:
Gas Density at STP:
Dalton's Law of Partial Pressure:
Collecting Gas Over Water:
_______________________________
Gas Density of Mixtures:
Average Kinetic Energy of a Gas:
Graham's Law of Effusion:
Kinetic Molecular Theory of Gases:
Gas Law Problems Review:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:18:18
0:18:18
 0:05:59
0:05:59
 0:01:58
0:01:58
 0:04:47
0:04:47
 0:03:31
0:03:31
 0:13:03
0:13:03
 0:05:18
0:05:18
 0:03:12
0:03:12
 0:10:41
0:10:41
 0:07:19
0:07:19
 0:13:08
0:13:08
 0:11:53
0:11:53
 0:21:07
0:21:07
 0:02:35
0:02:35
 0:07:51
0:07:51
 0:02:30
0:02:30
 0:04:01
0:04:01
 0:18:02
0:18:02
 0:04:06
0:04:06
 0:15:15
0:15:15
 0:04:20
0:04:20
 0:06:17
0:06:17
 0:07:25
0:07:25
 0:08:10
0:08:10