filmov
tv
HCl and CH3OH reaction mechanism

Показать описание
This video describes the mechanism for the reaction between hydrochloric acid and methanol, using standard arrows to explain the "electron pushing". The structures of the products are shown. The pKa values of the acid and the conjugate acid are compared to determine whether the reaction is reactant or product favored.
HCl and CH3OH reaction mechanism
Methanol + Hydrochloric Acid #reaction #science #chemistry
Reaction of CH3OH with HCl
Alkene + HBR + ROOR - Reaction Mechanism
Alkene Reactions
Alcohols 1 --- Methanol - CH3OH
Fischer Esterification of Benzoic Acid & Methanol in HCl (RXN Mechanism)
The reaction of D-ribose with one equivalent of methanol (CH3OH) plus HCl forms four products. Draw…...
Episode 13 Decode NCERT Alcohols Phenols and Ethers #puboard #chemistry
Grignard Reagent Reaction Mechanism
If 8.01g of CH3Br are produced when 4.051g of CH3OH is reacted with HCl what is the % yield?
Essential O-Chem 5 - Alkene Methanolysis
Mechanism of hydrogen chloride addition to alkene
Oxidation of Alcohols
Alkene Reactions #1 - Narrated Answer Key
Sodium Borohydride NaBH4 Reduction Reaction Mechanism
Benzoic Acid + Methanol = ?? (Methyl Benzoate + Water)
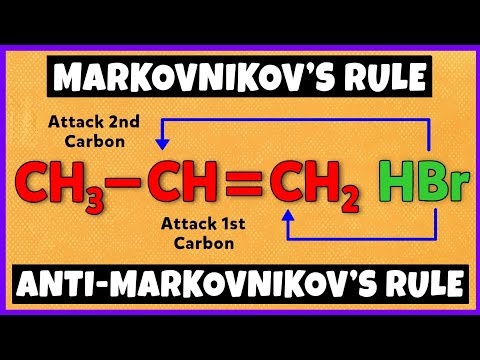
Markovnikov's Rule | Anti-Markovnikov's Rule | Mechanism
Markovnikov Addition of Methanol to an Alkene 001
reactions of alcohols with HX acids
Protecting Groups, Acetals, and Hemiacetals
Introduction to Reactions of Alcohols Ft. Professor Dave
11.12 | Compare the processes that occur when methanol (CH3OH), hydrogen chloride (HCl), and sodium
Substitution Reaction ( SN2 ) and Electrostatic Potential
Комментарии
 0:04:14
0:04:14
 0:00:16
0:00:16
 0:08:03
0:08:03
 0:07:17
0:07:17
 1:10:23
1:10:23
 0:00:07
0:00:07
 0:12:58
0:12:58
 0:00:33
0:00:33
 0:31:19
0:31:19
 0:12:50
0:12:50
 0:02:36
0:02:36
 0:04:11
0:04:11
 0:02:09
0:02:09
 0:16:48
0:16:48
 0:19:56
0:19:56
 0:07:32
0:07:32
 0:03:03
0:03:03
 0:12:48
0:12:48
 0:06:32
0:06:32
 0:03:56
0:03:56
 0:10:20
0:10:20
 0:03:03
0:03:03
 0:09:44
0:09:44
 0:00:08
0:00:08