filmov
tv
The Quantum Mechanical model of an atom. What do atoms look like? Why?

Показать описание
When Ernest Rutherford realized that atoms have a heavy nucleus, he hypothesized that the way the moon orbits earth is the same as the way an electron orbits the nucleus of atoms. We now know an atom would not look anything like this.
So what does an atom really look like? Why are atoms so small? Why doesn’t an electron fall onto the proton in the nucleus if they attract each other? Why can’t you squeeze an atom, if it is mostly empty space? It turns out it is not mostly empty space. And you may be shocked at what it is actually filled with.
The answers lie in the equations of quantum mechanics. One big problem with the Rutherford model is that when electrons are accelerated, as they would be in circular orbits, they create electromagnetic waves. This means that photons would be constantly radiated from the electron, causing it to lose energy.
So using classical laws for the atom just did not work. In 1900, Max Planck showed that matter emitted only discrete amounts of radiation, with energy E proportional to the frequency f – the proportionality constant h, being Planck’s constant.
Neil’s Bohr combined Rutherford’s model and Planck’s theory to hypothesize that the electron could exist in certain special orbits without radiating energy. Planck’s constant had units of angular momentum. So he hypothesized that only those orbits would be allowed where the angular momentum of the electron is quantized. He guessed that the lowest orbit would have the momentum h/2pi where the 2pi comes from the geometry of circular orbits. And any orbit could exist as long as it was an integer multiple of this number. But Bohr could not explain why these special orbits should exist in the first place.
French scientist, Louis de Broglie said if a particle has a momentum and a wavelength, then an electron is a wave. This required a huge philosophical leap, because he was said solid matter was composed of waves.
DeBroglie suggested that electrons can only exist in orbits where their waves interfere constructively, and that can only happen if the circumference of the orbit is equal to any integer times the wavelength. This explained why orbits would be at the radii that they are, something that Bohr could not do.
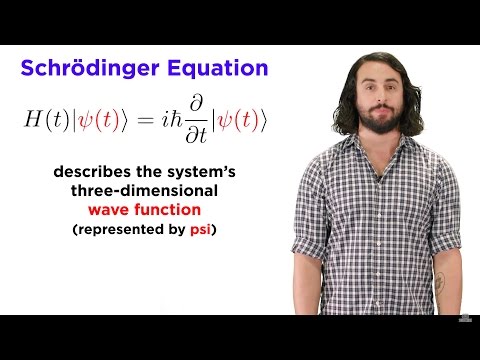
What is the nature of these waves? This puzzle was solved by Austrian physicist, Erwin Schrodinger. He said a wave can exist anywhere in 3D space, and formulated the rules to describe these waves in the Schrodinger equation. This equation could described the hydrogen atom with more detail and precision than the Bohr model, as well as all the other atoms in the periodic table. This was a new kind of mechanics - quantum mechanics.
Why can't we just look inside any material to see what atoms look like? The problem is that in order to be seen, the object has to be large enough to reflect light. But the largest atom is 1000 times smaller than a wavelength of visible light. So visible light just goes right through the atoms.
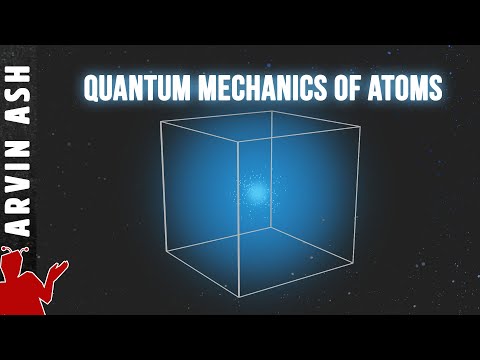
So we have to guess based on what the wave equation tells us. It tells us is that the electron forms a cloud around the nucleus. The shape of the cloud is governed by the wave function. The cloud represents the probable position of the electron if you were to measure it. The volume of the atom is thus not empty. It is filled everywhere with a cloud of electrons.
We can use the Schrodinger wave equation to find the electron probability. The highest probability occurs at 0.529 X 10^-10 meters, which is exactly the same radius calculated by Bohr. So the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics.
In Bohr’s model, the electron was assumed to be at this distance all the time, but in the Schrödinger model, it is never at any one radius. It has only the highest probability of being at this radius. The difference is due to the uncertainty principle, and the wave function.
And the same wave equation tells us that the nucleus of atoms is also a cloud. But the proton cloud is much smaller than the electron cloud because it is much more massive.
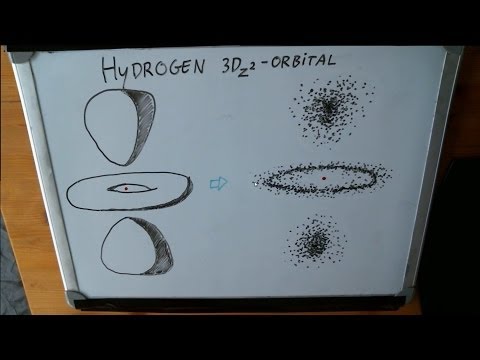
There are other shapes that the cloud of the hydrogen atoms could take as well, depending on the energy level and quantum state of the electron.
#whatdoatomslooklike
#atomicmodel
#quantummechanics
Why doesn’t the electron crash into the proton in the nucleus? Even if you drop an electron directly onto a proton, the electron will not fall and hit the proton - Because it would violate the Heisenberg uncertainty equation - both delta X and delta P would be zero. This can't be. Why can’t I squeeze two atoms together? It would require too much energy.
Link to paper showing picture of Hydrogen atom:
So what does an atom really look like? Why are atoms so small? Why doesn’t an electron fall onto the proton in the nucleus if they attract each other? Why can’t you squeeze an atom, if it is mostly empty space? It turns out it is not mostly empty space. And you may be shocked at what it is actually filled with.
The answers lie in the equations of quantum mechanics. One big problem with the Rutherford model is that when electrons are accelerated, as they would be in circular orbits, they create electromagnetic waves. This means that photons would be constantly radiated from the electron, causing it to lose energy.
So using classical laws for the atom just did not work. In 1900, Max Planck showed that matter emitted only discrete amounts of radiation, with energy E proportional to the frequency f – the proportionality constant h, being Planck’s constant.
Neil’s Bohr combined Rutherford’s model and Planck’s theory to hypothesize that the electron could exist in certain special orbits without radiating energy. Planck’s constant had units of angular momentum. So he hypothesized that only those orbits would be allowed where the angular momentum of the electron is quantized. He guessed that the lowest orbit would have the momentum h/2pi where the 2pi comes from the geometry of circular orbits. And any orbit could exist as long as it was an integer multiple of this number. But Bohr could not explain why these special orbits should exist in the first place.
French scientist, Louis de Broglie said if a particle has a momentum and a wavelength, then an electron is a wave. This required a huge philosophical leap, because he was said solid matter was composed of waves.
DeBroglie suggested that electrons can only exist in orbits where their waves interfere constructively, and that can only happen if the circumference of the orbit is equal to any integer times the wavelength. This explained why orbits would be at the radii that they are, something that Bohr could not do.
What is the nature of these waves? This puzzle was solved by Austrian physicist, Erwin Schrodinger. He said a wave can exist anywhere in 3D space, and formulated the rules to describe these waves in the Schrodinger equation. This equation could described the hydrogen atom with more detail and precision than the Bohr model, as well as all the other atoms in the periodic table. This was a new kind of mechanics - quantum mechanics.
Why can't we just look inside any material to see what atoms look like? The problem is that in order to be seen, the object has to be large enough to reflect light. But the largest atom is 1000 times smaller than a wavelength of visible light. So visible light just goes right through the atoms.
So we have to guess based on what the wave equation tells us. It tells us is that the electron forms a cloud around the nucleus. The shape of the cloud is governed by the wave function. The cloud represents the probable position of the electron if you were to measure it. The volume of the atom is thus not empty. It is filled everywhere with a cloud of electrons.
We can use the Schrodinger wave equation to find the electron probability. The highest probability occurs at 0.529 X 10^-10 meters, which is exactly the same radius calculated by Bohr. So the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics.
In Bohr’s model, the electron was assumed to be at this distance all the time, but in the Schrödinger model, it is never at any one radius. It has only the highest probability of being at this radius. The difference is due to the uncertainty principle, and the wave function.
And the same wave equation tells us that the nucleus of atoms is also a cloud. But the proton cloud is much smaller than the electron cloud because it is much more massive.
There are other shapes that the cloud of the hydrogen atoms could take as well, depending on the energy level and quantum state of the electron.
#whatdoatomslooklike
#atomicmodel
#quantummechanics
Why doesn’t the electron crash into the proton in the nucleus? Even if you drop an electron directly onto a proton, the electron will not fall and hit the proton - Because it would violate the Heisenberg uncertainty equation - both delta X and delta P would be zero. This can't be. Why can’t I squeeze two atoms together? It would require too much energy.
Link to paper showing picture of Hydrogen atom:
Комментарии
 0:04:36
0:04:36
 0:14:26
0:14:26
 0:08:24
0:08:24
 0:08:42
0:08:42
 0:11:19
0:11:19
 0:01:22
0:01:22
 0:05:35
0:05:35
 0:04:01
0:04:01
 0:42:12
0:42:12
 0:07:26
0:07:26
 0:01:10
0:01:10
 0:04:38
0:04:38
 0:10:52
0:10:52
 0:07:18
0:07:18
 0:08:45
0:08:45
 0:09:12
0:09:12
 0:06:28
0:06:28
 0:14:00
0:14:00
 0:07:47
0:07:47
 0:07:57
0:07:57
 0:12:16
0:12:16
 0:12:45
0:12:45
 0:09:42
0:09:42
 0:12:03
0:12:03