filmov
tv
Equilibrium Constant, Kc | A level Chemistry

Показать описание
A level Chemistry.
Physical Chemistry | Year 1
Explanation of Equilibrium constant and Kc calculations.
Physical Chemistry | Year 1
Explanation of Equilibrium constant and Kc calculations.
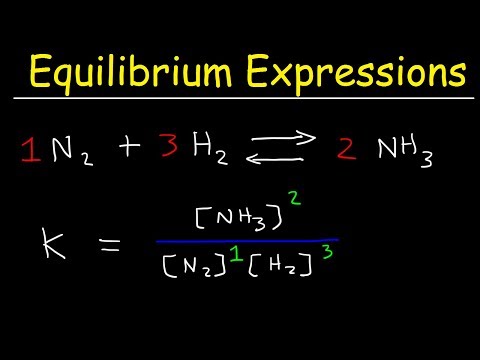
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Equilibrium Constant, Kc | A level Chemistry
AQA A-Level Chemistry - Equilibrium Constant, Kc
Chemical Equilibria and Reaction Quotients
The Equilibrium Constant
Equilibrium Constant: Kc | A-level Chemistry | OCR, AQA, Edexcel
Equilibrium Constant Grade 12: Exam
Kc UNITS || Equilibrium Constant Units || Kc Calculations || A level Chemistry
The equilibrium constant (Kc) Heterogeneous equilibria
Equilibrium Constant: Kc | A-Level Chemistry
How to write the equilibrium expression (Kc): 3 Trick Questions
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
15.3 Combining Equilibrium Constants
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
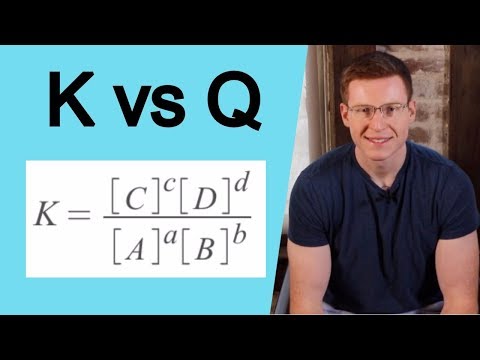
K (Equilibrium Constant) vs Q (Reaction Quotient)
Equilibrium Constant Kc | A level Chemistry | Question Walkthrough
Chemistry_Calculating the Equilibrium constant
Equilibrium Constant Kc - Exam Question Walkthrough|AQA A Level Chemistry
R2.3.2 / R2.3.3 Equilibrium constant, Kc
Kc and the equilibrium expression
Magnitude of the equilibrium constant | Equilibrium | AP Chemistry | Khan Academy
How to calculate the Equilibrium constant (Kc) Grade 12 - Explained in the simplest way
NEW VIDEO - Quick Revision - The Equilibrium Constant Kc
Комментарии
 0:53:22
0:53:22
 0:36:47
0:36:47
 0:19:23
0:19:23
 0:06:48
0:06:48
 0:06:15
0:06:15
 0:15:53
0:15:53
 0:06:48
0:06:48
 0:03:57
0:03:57
 0:17:25
0:17:25
 0:11:11
0:11:11
 0:04:32
0:04:32
 0:08:12
0:08:12
 0:06:46
0:06:46
 0:05:24
0:05:24
 0:28:41
0:28:41
 0:03:00
0:03:00
 0:11:51
0:11:51
 0:51:35
0:51:35
 0:20:14
0:20:14
 0:05:16
0:05:16
 0:05:24
0:05:24
 0:06:04
0:06:04
 0:11:30
0:11:30
 0:09:31
0:09:31