filmov
tv
Equilibrium Constant Kc | A level Chemistry | Question Walkthrough

Показать описание
Equilibrium Constant, Kc
Exam question walkthrough.
Question download:
A level Chemistry
Exam question walkthrough.
Question download:
A level Chemistry
Chemical Equilibria and Reaction Quotients
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
The Equilibrium Constant
AQA A-Level Chemistry - Equilibrium Constant, Kc
Equilibrium Constant, Kc | A level Chemistry
Equilibrium Constant Grade 12: Exam
Equilibrium Constant: Kc | A-level Chemistry | OCR, AQA, Edexcel
Equilibrium Constant: Kc | A-Level Chemistry
PHYSICAL SCIENCES GRADE 12 PAPER 2 REVISION PART 2
Using RICE to calculate equilibrium concentrations
Kc UNITS || Equilibrium Constant Units || Kc Calculations || A level Chemistry
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
Equilibrium Constant Kc | A level Chemistry | Question Walkthrough
How to write the equilibrium expression (Kc): 3 Trick Questions
How to Calculate Kc and Its Units || Chemical Equilibrium
Kc and the equilibrium expression
Magnitude of the equilibrium constant | Equilibrium | AP Chemistry | Khan Academy
Kc and its units | method to calculate the units of Kc | Unit of equilibrium constant
Equilibrium Made Easy: How to Solve Chemical Equilibrium Problems
9.1 Factors Affecting Equilibrium Constants
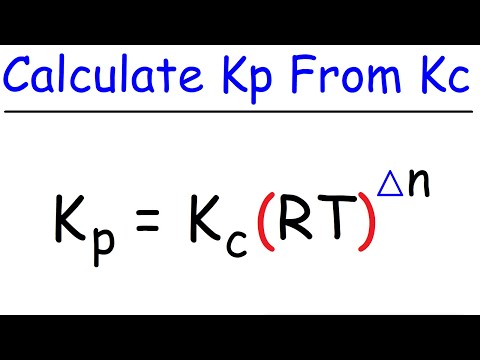
How To Calculate Kp From Kc - Chemical Equilibrium
Equilibrium Constant and it's Units
15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
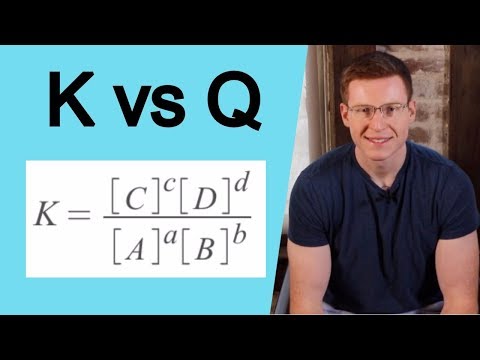
K (Equilibrium Constant) vs Q (Reaction Quotient)
Комментарии
 0:06:48
0:06:48
 0:53:22
0:53:22
 0:06:15
0:06:15
 0:19:23
0:19:23
 0:36:47
0:36:47
 0:06:48
0:06:48
 0:15:53
0:15:53
 0:11:11
0:11:11
 1:13:26
1:13:26
 0:10:13
0:10:13
 0:03:57
0:03:57
 0:08:12
0:08:12
 0:11:51
0:11:51
 0:04:32
0:04:32
 0:02:47
0:02:47
 0:05:24
0:05:24
 0:06:04
0:06:04
 0:12:25
0:12:25
 0:12:43
0:12:43
 0:06:00
0:06:00
 0:10:51
0:10:51
 0:19:36
0:19:36
 0:28:41
0:28:41
 0:03:00
0:03:00