filmov
tv
Radioactivity (11 of 16) Radioactive Decay Law, An Explanation
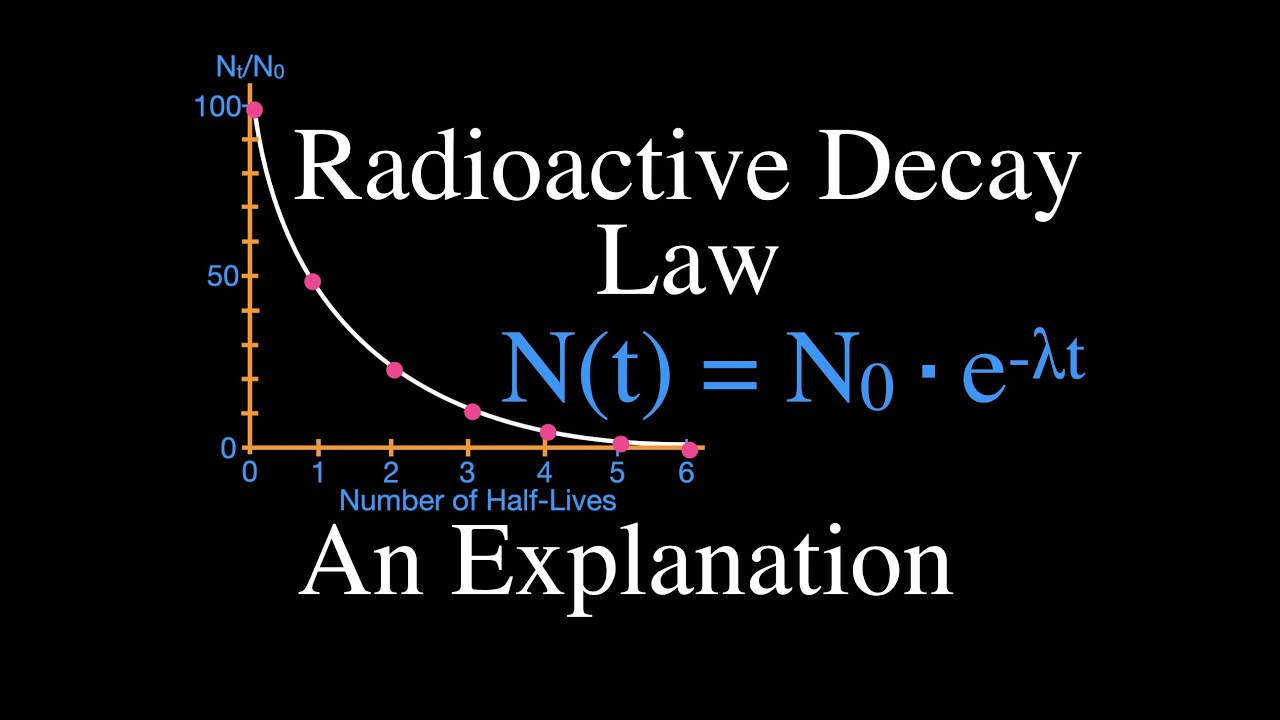
Показать описание
Explains what the radioactive decay law is. It states that the number of parent nuclei in a radioactive sample decreases exponentially over time. Includes a full explanation and a worked example for calculating the number of radioactive nuclei that remain after a specific amount of time. Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation in the form of alpha, beta and gamma particles.
Social Media for Step by Step Science:
Please support my channel by doing all of the following:
(1) Subscribe, get all my physics, chemistry and math videos.
(2) Give me a thumbs up for this video.
(3) Leave me a nice positive comment.
(4) Sharing is Caring, sharing this video with all of your friends.
Social Media for Step by Step Science:
Please support my channel by doing all of the following:
(1) Subscribe, get all my physics, chemistry and math videos.
(2) Give me a thumbs up for this video.
(3) Leave me a nice positive comment.
(4) Sharing is Caring, sharing this video with all of your friends.
Radioactivity (11 of 16) Radioactive Decay Law, An Explanation
GCSE Physics - Alpha, Beta and Gamma Radiation #33
11. Radioactivity and Series Radioactive Decays
Types of Nuclear Radiation
Half life | Radioactivity | Physics | FuseSchool
What is Radioactivity and Is It Always Harmful: Explained in Really Simple Words
Radioactivity (1 of 16) An Explanation
Radioactivity (3 of 16) Three Types of Radioactive Decay, An Explanation
Radioactivity: What is a Decay Chain
Physics:Grade 12 ES (Radioactivity )
radioactivity explained
What is radioactivity and half-life? | Nuclear Physics | Visual Explanation
Radioactivity - Radioactive Dating - Using Half Life to find the age of objects.
The Most Radioactive Places on Earth
Nuclear Chemistry (Radioactivity) - NC 01
Touching mercury
27. Nuclear Materials — Radiation Damage and Effects in Matter
Lead is not that bad #radiation #protection
Radioactivity (JAMB CHEMISTRY) | Types of Radiation | Alpha & Beta Decay | Nuclear Fission &...
Radioactivity (12 of 16) Radioactive Decay Law, Examples Problems
Radioactive Decay/Nuclear Radiation (Alpha, Beta, Gamma) - GCSE Physics Revision
Hawking radiation
Physics Grade 10 & 11, Radioactivity
Radioactivity (8 of 16) Decay Activity, An Explanation
Комментарии
 0:10:36
0:10:36
 0:04:37
0:04:37
 0:54:11
0:54:11
 0:09:23
0:09:23
 0:04:54
0:04:54
 0:08:08
0:08:08
 0:12:14
0:12:14
 0:14:13
0:14:13
 0:17:16
0:17:16
 0:17:08
0:17:08
 0:25:07
0:25:07
 0:04:42
0:04:42
 0:03:42
0:03:42
 0:11:17
0:11:17
 0:27:29
0:27:29
 0:00:39
0:00:39
 0:55:54
0:55:54
 0:01:01
0:01:01
 0:52:57
0:52:57
 0:12:09
0:12:09
 0:04:46
0:04:46
 0:16:30
0:16:30
 0:05:11
0:05:11
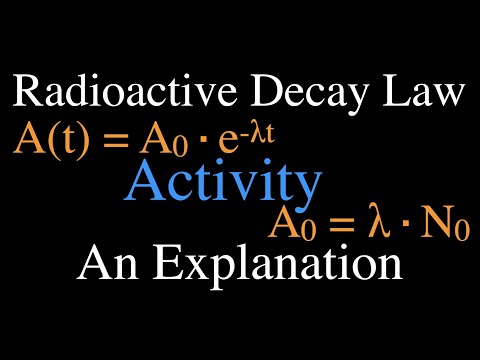 0:11:07
0:11:07