filmov
tv
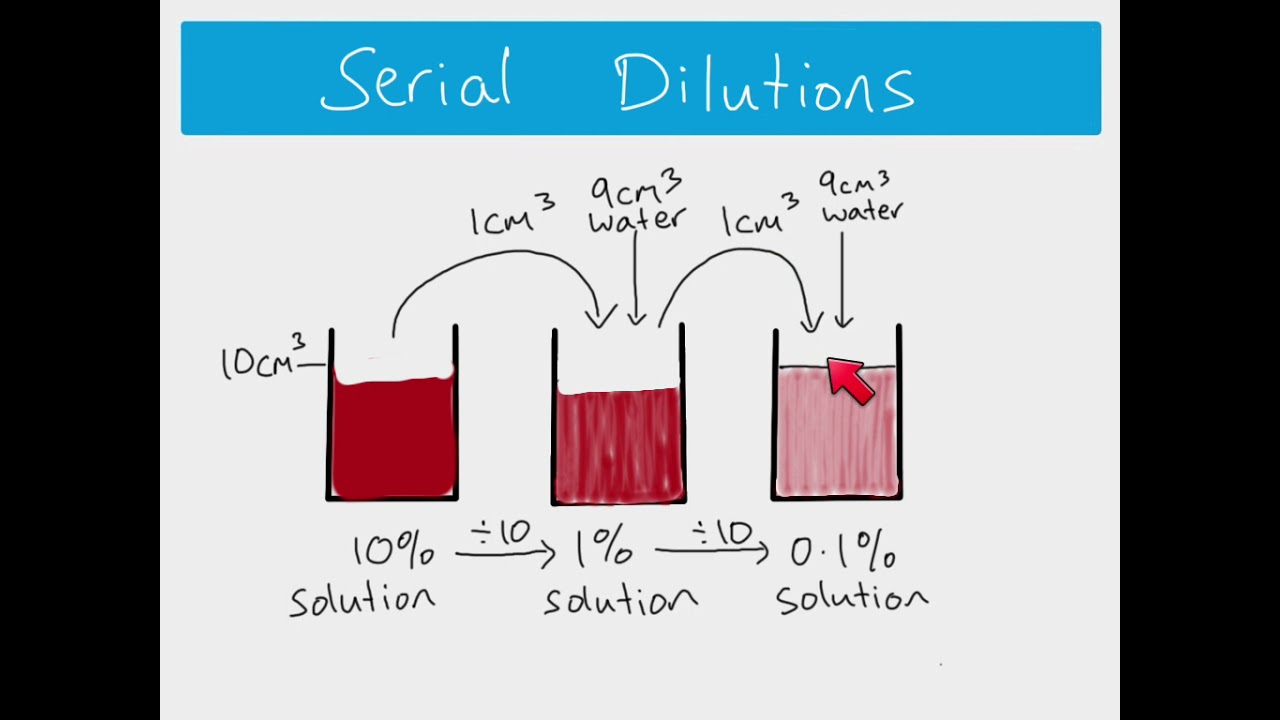
AS Biology - How to calculate serial and simple dilutions
Показать описание
AS Biology - Biochemistry topic. How to calculate serial dilutions and simple dilutions
AS Biology - How to calculate serial and simple dilutions
AS Biology - How to calibrate a microscope
Try this to get an A* in Biology
How I got an A* in A Level Biology. (the struggle) || Revision Tips, Resources and Advice!
BIOLOGY explained in 17 Minutes
Serial Dilution | Required Practical Revision for Biology and Chemistry A-Level
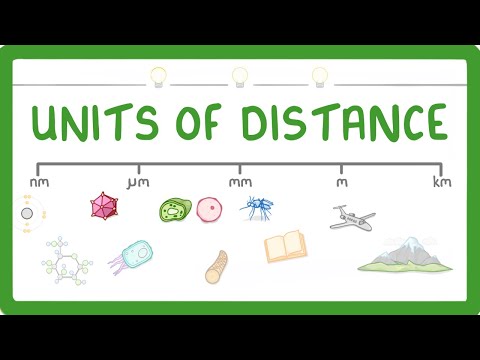
GCSE Biology - How to convert between nm, um, mm, m and km #4
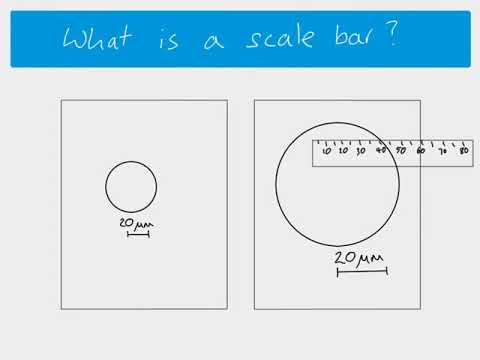
AS Biology - Using scale bars to calculate magnification
AP Bio 4.7 (regulation of the cell cycle) in a minute! #apbiology #apbio #biology
All of Biology in 9 minutes
A-Level Biology - Perfect Biological Drawings in Paper 3
How to EASILY score A+ for ALL SCIENCE SPM + NOTES | Biology, Chemistry, Physics
Chapter 1.2 - Micrometry and Magnification | Cambridge A-Level 9700 Biology
Advice for Starting A Level Biology & My HONEST Experience // UnJaded Jade
Science Raps: GCSE Biology - Heart Structure
How To Get an A in Biology
A level biology: How to calculate heart rate
How to get FULL MARKS in Biology GCSE 📚| Answer Questions with Me 📝 (Get a GRADE 9)
Chapter 1.1 - Microscopy | Cambridge A-Level 9700 Biology
Detailed & Honest Experience of A level Biology + Advice & Tips 🌵
How I STUDY for my Biology Classes | Biomedical Science Major
Biology A-level 2025 exams 2025. AQA paper 1 (or ENTIRE AS LEVEL) -Learn all the theory for the exam
How To Use a Potometer - Biology GCSE | kayscience.com
Osmosis - Biology A-level Required Practical
Комментарии
 0:09:16
0:09:16
 0:13:10
0:13:10
 0:00:15
0:00:15
 0:10:45
0:10:45
 0:17:31
0:17:31
 0:04:24
0:04:24
 0:06:20
0:06:20
 0:07:14
0:07:14
 0:01:00
0:01:00
 0:09:31
0:09:31
 0:04:49
0:04:49
 0:09:04
0:09:04
 0:23:26
0:23:26
 0:08:33
0:08:33
 0:00:37
0:00:37
 0:05:32
0:05:32
 0:03:47
0:03:47
 0:23:06
0:23:06
 0:24:03
0:24:03
 0:14:13
0:14:13
 0:13:34
0:13:34
 3:09:17
3:09:17
 0:05:38
0:05:38
 0:11:40
0:11:40