filmov
tv
Power Cycle Thermal Efficiency – Impossible, Reversible, or Irreversible?

Показать описание
To calculate the thermal efficiency of a system that undergoes a power cycle, you may first want to determine whether the cycle operates reversibly, irreversibly, or is impossible.
These reversible, irreversible, impossible type problems are found in the 2nd Law aspects of power cycles interacting with two reservoirs section in Fundamentals of Engineering Thermodynamics Moran chapter 5. They often start with the phrase “an inventor claims to have developed a power cycle” and you are tasked with finding the maximum performance measures for cycles operating between two reservoirs, then checking whether the claimed performance is lower, equal, or higher than the Carnot maximum.
Thermodynamics chapter 5 covers refrigeration cycles, heat pump cycles, and a power cycle operating between hot and cold reservoirs. This video only addresses the power cycle, but refrigeration and heat pump analysis is nearly identical, just swapping out the appropriate efficiency equation.
TIMECODES
0:00 2nd Law of Thermodynamics
1:30 Irreversible vs Reversible vs Impossible
5:00 Use efficiency to find minimum Qc
These reversible, irreversible, impossible type problems are found in the 2nd Law aspects of power cycles interacting with two reservoirs section in Fundamentals of Engineering Thermodynamics Moran chapter 5. They often start with the phrase “an inventor claims to have developed a power cycle” and you are tasked with finding the maximum performance measures for cycles operating between two reservoirs, then checking whether the claimed performance is lower, equal, or higher than the Carnot maximum.
Thermodynamics chapter 5 covers refrigeration cycles, heat pump cycles, and a power cycle operating between hot and cold reservoirs. This video only addresses the power cycle, but refrigeration and heat pump analysis is nearly identical, just swapping out the appropriate efficiency equation.
TIMECODES
0:00 2nd Law of Thermodynamics
1:30 Irreversible vs Reversible vs Impossible
5:00 Use efficiency to find minimum Qc
Heat Engines, Thermal Efficiency, & Energy Flow Diagrams - Thermodynamics & Physics Problems
Ideal BRAYTON CYCLE Explained in 11 Minutes!
Carnot Cycle & Heat Engines, Maximum Efficiency, & Energy Flow Diagrams Thermodynamics &am...
Power Cycle Thermal Efficiency – Impossible, Reversible, or Irreversible?
Thermodynamics Lecture 24: Rankine Cycle
Basics of Power Plant Engineering, L2: Rankine-cycle thermal efficiency
Rankine Cycle Efficiency and Net Power Output Calculations
Calculating Isentropic Efficiency of a Turbine in 2 Minutes!
Carnot Cycle - PV diagram, efficiency derivation and limitations [Hindi] #carnotcycle #carnot #cycle
Thermodynamics - Power and Refrigeration Cycles and Thermal Efficiency
Thermodynamics Lecture 31: Brayton Cycle
Brayton Cycle
Thermodynamics & Power Plant - GATE Exercise 2
ENGR251: The Rankine cycle / Example
Rankine Cycle - Steam Power Plant
MECH351: Combined cycles (Brayton cycle + Rankine cycle)
Mechanical Engineering Thermodynamics - Lec 19, pt 2 of 5: Ideal Rankine Cycle
Coal Steam Power Plant Efficiency Example - Thermodynamics - in 2 Minutes!
2nd Law Intro and POWER CYCLES in 10 Minutes!
Otto Cycle
Rankine Cycle
Simple Ideal Rankine Cycle | Coal Nuclear Power Plant - Example 10.1
MECH351: Increase Rankine cycle efficiency
Heat Engines, Refrigerators, & Cycles: Crash Course Engineering #11
Комментарии
 0:21:10
0:21:10
 0:11:19
0:11:19
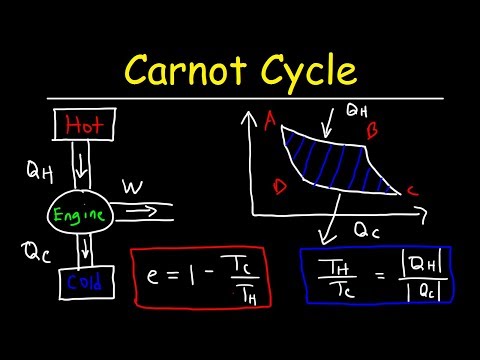 0:20:17
0:20:17
 0:06:56
0:06:56
 0:09:45
0:09:45
 0:13:14
0:13:14
 0:22:59
0:22:59
 0:02:17
0:02:17
 0:08:27
0:08:27
 0:19:30
0:19:30
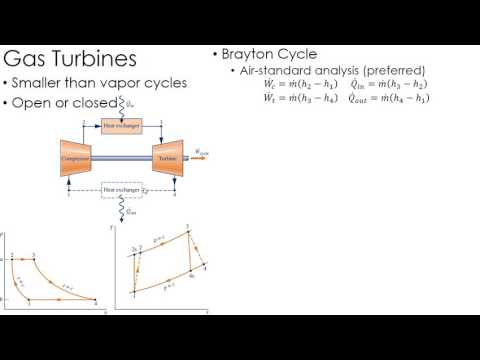 0:04:31
0:04:31
 0:09:05
0:09:05
 0:10:42
0:10:42
 0:37:31
0:37:31
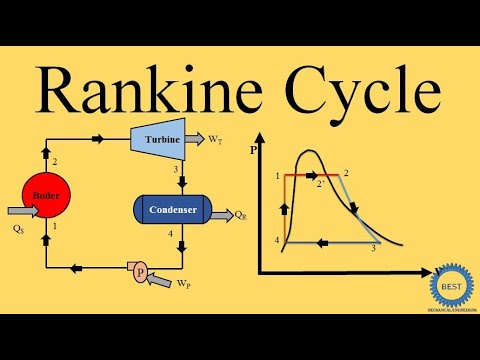 0:16:56
0:16:56
 0:04:51
0:04:51
 0:10:54
0:10:54
 0:01:36
0:01:36
 0:09:55
0:09:55
 0:09:30
0:09:30
 0:08:14
0:08:14
 0:26:37
0:26:37
 0:06:12
0:06:12
 0:10:44
0:10:44