filmov
tv
Heisenberg Uncertainty Principle

Показать описание
Heisenberg's Uncertainty Principle Made Easy!
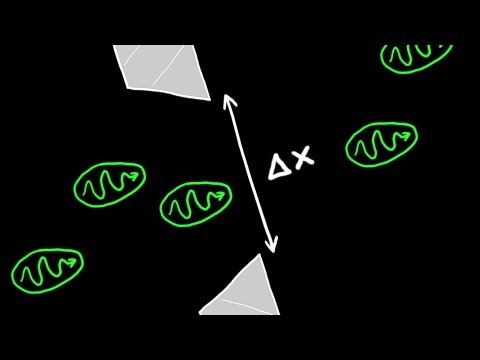
Heisenberg's Uncertainty Principle is a fundamental concept in quantum mechanics, formulated by the German physicist Werner Heisenberg in 1927. The principle states that there is a limit to the precision with which certain pairs of properties, such as the position and momentum of a particle, can be simultaneously known.
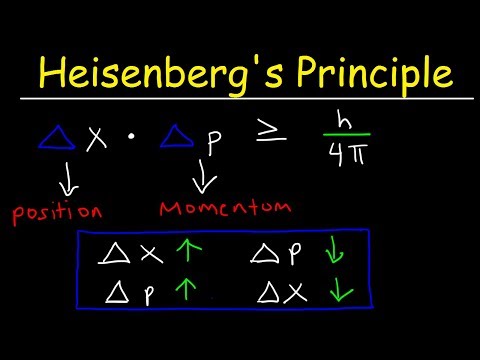
Mathematically, the Uncertainty Principle is often expressed as follows for position (Δx) and momentum (Δp) of a particle:
Δx * Δp ≥ ħ / 2
where:
- Δx is the uncertainty in position,
- Δp is the uncertainty in momentum,
- ħ (h-bar) is the reduced Planck constant, approximately equal to 1.054 x 10^-34 J·s.
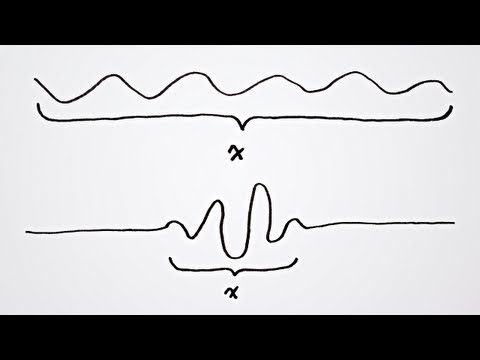
This principle implies that the more precisely one property (e.g., position) is known, the less precisely the other property (e.g., momentum) can be known, and vice versa. In other words, there is a fundamental limit to the precision with which certain pairs of conjugate variables can be simultaneously measured. This inherent uncertainty is a fundamental aspect of the behavior of particles at the quantum level and challenges our classical intuition about the determinism of physical systems.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Heisenberg's Uncertainty Principle is a fundamental concept in quantum mechanics, formulated by the German physicist Werner Heisenberg in 1927. The principle states that there is a limit to the precision with which certain pairs of properties, such as the position and momentum of a particle, can be simultaneously known.
Mathematically, the Uncertainty Principle is often expressed as follows for position (Δx) and momentum (Δp) of a particle:
Δx * Δp ≥ ħ / 2
where:
- Δx is the uncertainty in position,
- Δp is the uncertainty in momentum,
- ħ (h-bar) is the reduced Planck constant, approximately equal to 1.054 x 10^-34 J·s.
This principle implies that the more precisely one property (e.g., position) is known, the less precisely the other property (e.g., momentum) can be known, and vice versa. In other words, there is a fundamental limit to the precision with which certain pairs of conjugate variables can be simultaneously measured. This inherent uncertainty is a fundamental aspect of the behavior of particles at the quantum level and challenges our classical intuition about the determinism of physical systems.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Комментарии
 0:04:44
0:04:44
 0:04:12
0:04:12
 0:17:18
0:17:18
 0:11:59
0:11:59
 0:01:04
0:01:04
 0:06:11
0:06:11
 0:11:02
0:11:02
 0:09:58
0:09:58
 0:21:23
0:21:23
 1:01:57
1:01:57
 0:09:20
0:09:20
 0:14:52
0:14:52
 0:09:22
0:09:22
 0:19:21
0:19:21
 0:14:43
0:14:43
 0:06:07
0:06:07
 0:14:35
0:14:35
 0:03:55
0:03:55
 0:05:20
0:05:20
 0:08:02
0:08:02
 0:14:49
0:14:49
 1:04:07
1:04:07
 0:17:18
0:17:18
 0:03:17
0:03:17