filmov
tv
Heisenberg's Uncertainty Principle Explained & Simplified - Position & Momentum - Chemistry Problems

Показать описание
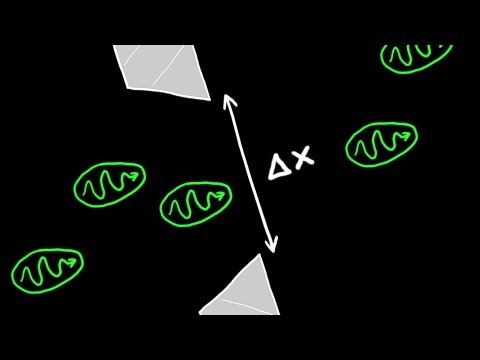
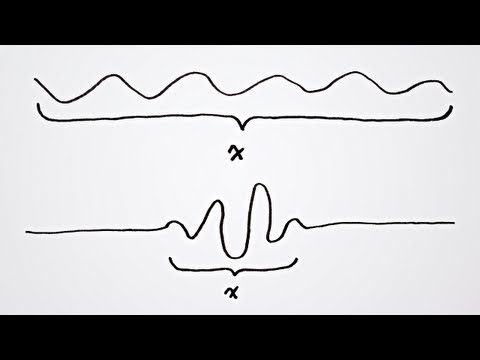
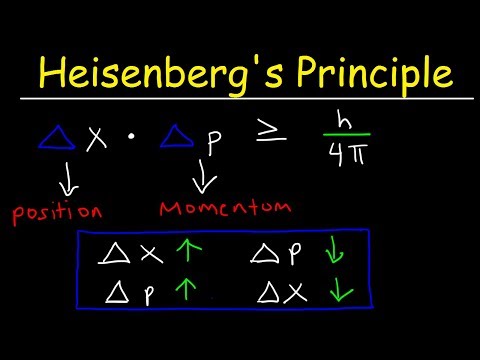
This chemistry video tutorial explains the concept of heisenberg's uncertainty principle in a simplified way. His principle applies mostly for small particles and events. As the uncertainty in the position of a particle increases, the uncertainty in momentum decreases. This video contains some math practice problems that illustrate Heisenberg's uncertainty principle.
Atomic Theory - Formula Sheet:
Speed of Light, Frequency, Wavelength:
Photon Energy:
The Photoelectric Effect:
De Broglie Wavelength:
The Bohr Model of Hydrogen:
Heisenberg's Uncertainty Principle:
________________________________
Intro to Quantum Numbers:
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Intro to Electron Configuration:
Electron Configuration Exceptions:
Noble Gas Notation:
Electron Configuration of Ions:
_______________________________
Orbital Diagrams:
Paired & Unpaired Electrons:
Aufbau's Principle & Hund's Rule:
Paramagnetic & Diamagnetic Elements:
Valence Electrons & Periodic Table:
Effective Nuclear Charge:
_________________________________
Slater's Rule:
How To Identify The Element:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Atomic Theory - Formula Sheet:
Speed of Light, Frequency, Wavelength:
Photon Energy:
The Photoelectric Effect:
De Broglie Wavelength:
The Bohr Model of Hydrogen:
Heisenberg's Uncertainty Principle:
________________________________
Intro to Quantum Numbers:
Orbitals & Atomic Energy Levels:
Maximum Number of Electrons:
Intro to Electron Configuration:
Electron Configuration Exceptions:
Noble Gas Notation:
Electron Configuration of Ions:
_______________________________
Orbital Diagrams:
Paired & Unpaired Electrons:
Aufbau's Principle & Hund's Rule:
Paramagnetic & Diamagnetic Elements:
Valence Electrons & Periodic Table:
Effective Nuclear Charge:
_________________________________
Slater's Rule:
How To Identify The Element:
Quantum Numbers - Mega Review:
Quantum Numbers - Practice Test:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:04:12
0:04:12
 0:04:44
0:04:44
 0:06:11
0:06:11
 0:09:20
0:09:20
 0:14:43
0:14:43
 0:06:07
0:06:07
 0:09:58
0:09:58
 0:01:04
0:01:04
 0:11:59
0:11:59
 0:11:02
0:11:02
 0:21:23
0:21:23
 0:17:18
0:17:18
 1:01:57
1:01:57
 0:06:13
0:06:13
 0:00:59
0:00:59
 0:14:52
0:14:52
 0:09:22
0:09:22
 0:16:14
0:16:14
 0:01:00
0:01:00
 0:14:35
0:14:35
 0:08:55
0:08:55
 0:02:16
0:02:16
 0:09:24
0:09:24
 1:04:07
1:04:07