filmov
tv
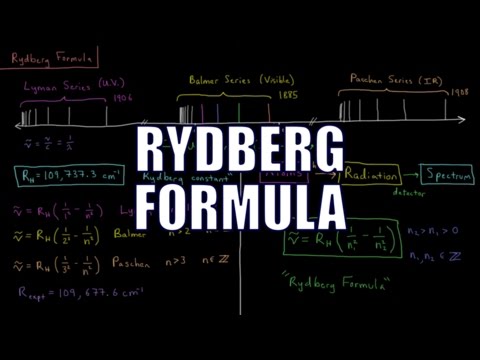
Using the Rydberg Equation to Solve Transitions Between Energy Levels

Показать описание
This video covers:
1. Atomic Spectroscopy and the Bohr Model
2. Electromagnetic Radiation
3. Particle Nature of Light
4. Doing Calculations
1. Atomic Spectroscopy and the Bohr Model
2. Electromagnetic Radiation
3. Particle Nature of Light
4. Doing Calculations
Quantum Chemistry 1.3 - Rydberg Formula
Balmer Series and Rydberg Equation | Professor Adam Teaches
Rydberg Equation and Balmer Series of Hydrogen
27: Using the Rydberg equation
Emission spectrum of hydrogen | Chemistry | Khan Academy
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Using the Rydberg Equation to Solve Transitions Between Energy Levels
Bohr Model of the Hydrogen Atom
27a: Using the Rydberg equation
Using the Rydberg Equation 002 - Paschen Series
Using the Rydberg Equation 006 - Calculate the Excitation Energy
Rydberg Formula Derivation (Bohr Model Energy Levels and Photon Emission)
Using the Rydberg Equation 005 - Calculate the Emission Energy 002
Using the Rydberg Equation 001 - Balmer Series
Emission and Absorption - Rydberg Equation
Calculations with the Bohr, Planck and Rydberg Equations
How to find starting or final values using the Rydberg Equation
Chemistry: Using the Rydberg Equation
Use the Rydberg equation to calculate the frequency of a photon absorbed when the hydrogen atom und…...
How to solve a Rydberg/Balmer problem with solution
Lyman series using Rydberg's formula for the wavelengths: first four wavelengths in the Lyman s...
Rydberg’s Equation 🤟✅ #equation #formula1 #chemistry
Solving problems using the Rydberg equation
Using the Bohr Rydberg equation on Hydrogen
Комментарии
 0:06:26
0:06:26
 0:04:56
0:04:56
 0:04:57
0:04:57
 0:08:56
0:08:56
 0:10:50
0:10:50
 0:21:44
0:21:44
 0:08:47
0:08:47
 0:04:50
0:04:50
 0:17:45
0:17:45
 0:05:31
0:05:31
 0:03:46
0:03:46
 0:09:19
0:09:19
 0:05:46
0:05:46
 0:06:08
0:06:08
 0:07:11
0:07:11
 0:05:30
0:05:30
 0:13:30
0:13:30
 0:03:01
0:03:01
 0:00:33
0:00:33
 0:02:49
0:02:49
 0:03:18
0:03:18
 0:00:24
0:00:24
 0:18:48
0:18:48
 0:10:50
0:10:50