filmov
tv
Using the Rydberg Equation 002 - Paschen Series

Показать описание
What is the shortest wavelength line (nm) in the Paschen series (n2 = 3, n1 = ∞) for hydrogen?
Using the Rydberg Equation 002 - Paschen Series
Using the Rydberg Equation 005 - Calculate the Emission Energy 002
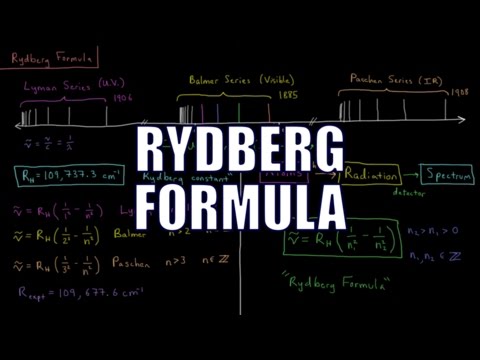
27: Using the Rydberg equation
27a: Using the Rydberg equation
Applying the Rydberg equation to the hydrogen atom emission spectra Su 2 A1
Quantum Chemistry 1.3 - Rydberg Formula
Using the Rydberg Equation to Solve Transitions Between Energy Levels
Balmer Series and Rydberg Equation | Professor Adam Teaches
Lecture 37 - Chapter 2 - Rydberg Equation
2.1 PART 3 - The Rydberg Equation
Rydberg Equation and Balmer Series of Hydrogen
How small are atoms?
Bohr Model of the Hydrogen Atom
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Using the Bohr Rydberg equation on Hydrogen
RYDBERG EQUATION - Calculating the Energy of the Shells in a Li(+2) Ion
Using the Rydberg Equation 006 - Calculate the Excitation Energy
Rydberg Formula Derivation (Bohr Model Energy Levels and Photon Emission)
Lesson 1A 2 Rydberg Formula
Chemistry: Using the Rydberg Equation
Using the Rydberg Equation 001 - Balmer Series
Week 4 Chemistry Bohr’s formula & Rydberg equation Lesson 2.1e SDS
Emission spectrum of hydrogen | Chemistry | Khan Academy
2 1 Bohr's Atomic Model until Rydberg Equation
Комментарии
 0:05:31
0:05:31
 0:05:46
0:05:46
 0:08:56
0:08:56
 0:17:45
0:17:45
 0:21:25
0:21:25
 0:06:26
0:06:26
 0:08:47
0:08:47
 0:04:56
0:04:56
 0:10:04
0:10:04
 0:18:58
0:18:58
 0:04:57
0:04:57
 0:00:48
0:00:48
 0:04:50
0:04:50
 0:21:44
0:21:44
 0:10:50
0:10:50
 0:02:42
0:02:42
 0:03:46
0:03:46
 0:09:19
0:09:19
 0:11:24
0:11:24
 0:03:01
0:03:01
 0:06:08
0:06:08
 0:08:38
0:08:38
 0:10:50
0:10:50
 0:55:52
0:55:52