filmov
tv
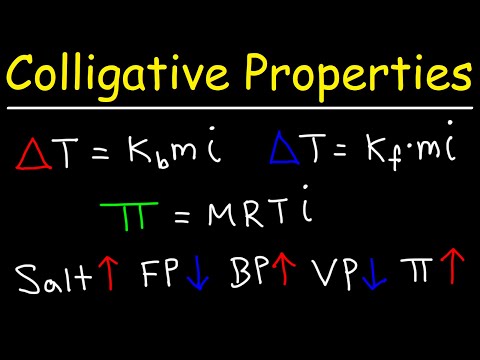
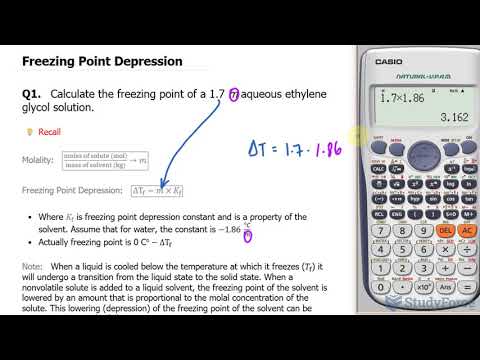
Calculate the depression in the freezing point of water when 10g of CH3CH2CHClCOOH is added to 250g

Показать описание
Calculate the depression in the freezing point of water when 10g of CH3CH2CHClCOOH is added to 250g of water. Ka = 1.4 x 1o-3 Kg = 1.86 K kg mol-1.
 0:01:22
0:01:22
 0:10:11
0:10:11
 0:07:33
0:07:33
 0:08:36
0:08:36
 0:02:44
0:02:44
 0:01:00
0:01:00
 0:03:29
0:03:29
 0:21:00
0:21:00
 0:54:59
0:54:59
 0:25:23
0:25:23
 0:04:53
0:04:53
 0:01:00
0:01:00
 0:09:47
0:09:47
 0:02:53
0:02:53
 0:09:08
0:09:08
 0:02:37
0:02:37
 0:06:12
0:06:12
 0:04:41
0:04:41
 0:02:35
0:02:35
 0:10:19
0:10:19
 0:15:16
0:15:16
 0:00:35
0:00:35
 0:06:56
0:06:56
 0:10:34
0:10:34