filmov
tv
Calculating Molar Mass from Freezing Point Depression

Показать описание
This short video shows you how to calculate the molar mass of a nonelectrolyte (van't hoff=1) using freezing point depression.
Example Question:
The freezing point of a solution that contains 1.00 g of an unknown compound, dissolved in 10.0 g of benzene is found to be 2.07oC. The freezing point of pure benzene is 5.48 oC. The molal freezing point depression constant of benzene is 5.12 oC /molal. What is the molecular weight of the unknown compound?
Example Question:
The freezing point of a solution that contains 1.00 g of an unknown compound, dissolved in 10.0 g of benzene is found to be 2.07oC. The freezing point of pure benzene is 5.48 oC. The molal freezing point depression constant of benzene is 5.12 oC /molal. What is the molecular weight of the unknown compound?
Calculating Molar Mass from Freezing Point Depression
Calculating molar mass from freezing point depression
solving for molar mass from freezing point depression
ALEKS: Using a solution freezing point to calculate molar mass
Determining Molar Mass of Unknown using Freezing Point Depression (Colligative Properties)
General Chemistry II - Freezing Point Depression - Solving for Molar Mass
Experiment 12: Determining Molar Mass by Freezing Point Depression
ALEKS - Using a Solution Freezing Point to Calculate a Molar Mass
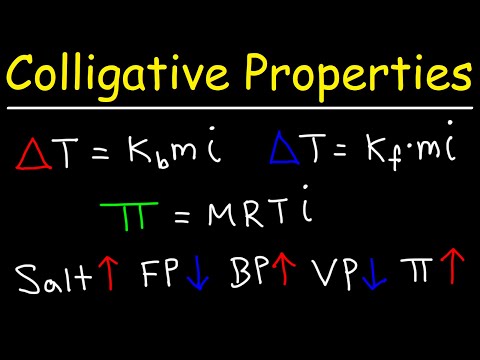
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
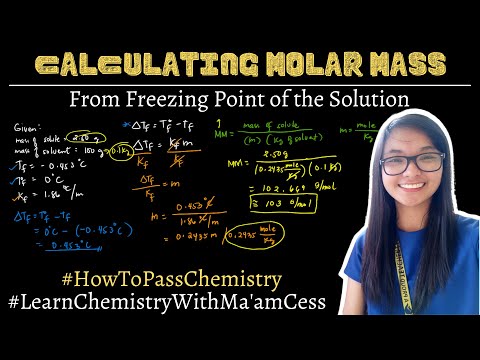
How to calculate molar mass from freezing point of the solution | Learn Chemistry with Ma'am Ce...
Calculating The Molar Mass Of An Unknown Compound From Freezing Point Depression
Calculating Molar Mass from Boiling Point Elevation
Calc Molar Mass from freezing point depression Problem 449
CHEM1520L Experiment 003 Molar Mass Determination from Freezing Point Depression
Freezing Point Depression (HD)
Molar mass calculation using freezing point depression data
Week 4 - 4. Calculating molar mass from boiling point elevation
DETERMINATION OF MOLAR MASS BY FREEZING POINT DEPRESSION
Calculate molar mass of unknown solute by freezing point depression
Molar Mass by Freezing Point.wmv
Determining Molar Mass From Freezing Point
CHM 112 Molar mass by Freezing Point Depression
Molar Mass by Freezing Point Depression Lab
How to Calculate Molar Concentration ☑️😁 #science #chemistry #shorts
Комментарии
 0:02:44
0:02:44
 0:02:15
0:02:15
 0:03:37
0:03:37
 0:05:35
0:05:35
 0:04:41
0:04:41
 0:03:37
0:03:37
 0:15:16
0:15:16
 0:09:50
0:09:50
 0:25:23
0:25:23
 0:11:28
0:11:28
 0:06:56
0:06:56
 0:02:30
0:02:30
 0:06:32
0:06:32
 0:18:31
0:18:31
 0:04:59
0:04:59
 0:05:33
0:05:33
 0:05:05
0:05:05
 0:02:43
0:02:43
 0:12:17
0:12:17
 0:10:50
0:10:50
 0:07:15
0:07:15
 0:11:58
0:11:58
 0:25:17
0:25:17
 0:00:58
0:00:58