filmov
tv
Huckel's rules for aromatic rings

Показать описание
Huckel's rules describe four criteria for a molecule to be considered aromatic. (1) The molecule needs a ring. (2) The ring atoms must lie in a plane - a flat ring. (3) Every ring atom needs a p orbital. In short, this normally means that every ring atom must be sp2 hybridized. (4) The number of electrons in the ring atom p orbitals must satisfy the equation - # electrons = 4n +2 - so that n equals a non-negative positive integer (i.e., 0, 1, 2, etc.). We can apply Huckel's rule to aromatic molecules like furan, pyridine, and benzene.
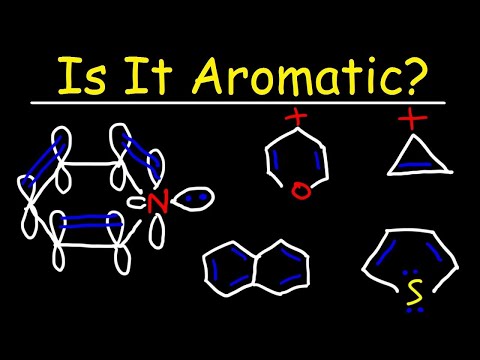 0:10:43
0:10:43
 0:10:00
0:10:00
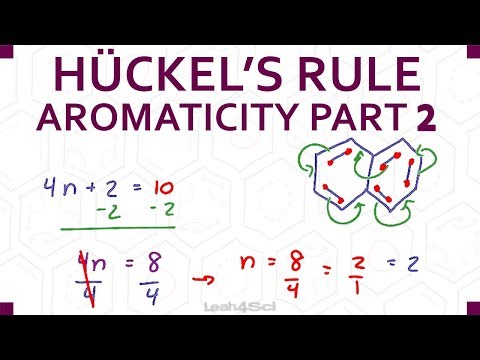 0:11:00
0:11:00
 0:04:54
0:04:54
 0:00:38
0:00:38
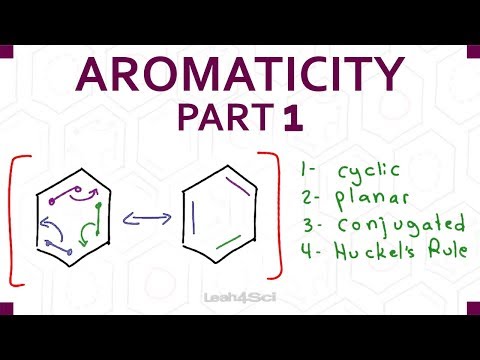 0:10:12
0:10:12
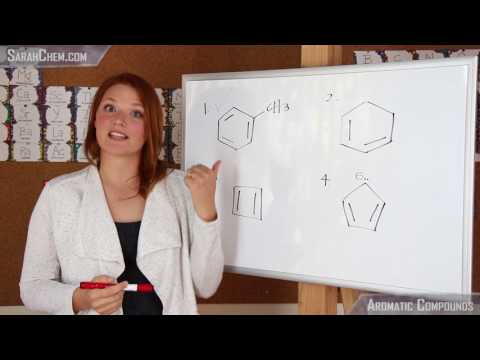 0:03:58
0:03:58
 0:04:26
0:04:26
 0:22:22
0:22:22
 0:03:30
0:03:30
 0:10:24
0:10:24
 0:11:26
0:11:26
 0:14:05
0:14:05
 0:11:08
0:11:08
 0:00:51
0:00:51
 0:05:55
0:05:55
 0:05:07
0:05:07
 0:15:35
0:15:35
 0:00:58
0:00:58
 0:00:11
0:00:11
 0:05:41
0:05:41
 0:11:04
0:11:04
 0:31:59
0:31:59
 0:00:51
0:00:51